

十六烷基三羟乙基溴化铵促进1-辛烯氢甲酰化水/有机两相反应研究
收稿日期: 2013-02-01
网络出版日期: 2013-03-21
基金资助
项目受四川大学青年教师科研启动基金(No. 2011SCU11084)和中国石油天然气股份有限公司石油化工研究院(No. 2011B-2606)资助.
Study on 1-Octene Hydroformylation Promoted by Cetyltrihydroxyethyl Ammonium Bromide in Aqueous/Organic Biphasic Solution
Received date: 2013-02-01
Online published: 2013-03-21
Supported by
Project supported by Sichuan University Scientific Research Foundation for Young Teachers (No. 2011SCU11084) and Petrochemical Research Institute of China National Petroleum Corporation (No. 2011B-2606).
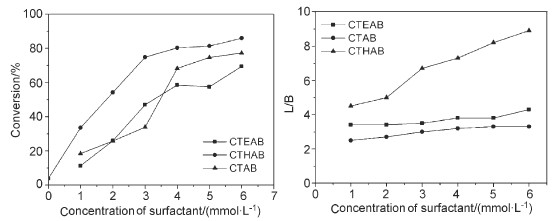
研究了水/有机两相体系中表面活性剂十六烷基三羟乙基溴化铵(CTHAB)对Rh/TPPTS (TPPTS: 三[间-磺酸钠基苯基]膦)催化的1-辛烯氢甲酰化反应的促进作用, 初步证实了CTHAB分子中的羟基可与催化活性物种的铑之间发生配位. 与传统表面活性剂十六烷基三甲基溴化铵(CTAB)相比, 表面活性剂CTHAB的添加不仅加速了水/有机两相1-辛烯氢甲酰化反应, 而且提高了成醛的正/异比, 促进作用明显. 在 [Rh]=0.8 mmol/L, [TPPTS]/[Rh]=40, [CTHAB]=4.0 mmol/L, 90 ℃, 0.5 MPa, 1.5 h时, 生成醛的TOF为497 h-1, 正/异比(L/B)可达25.6. 催化剂经7次循环后, 反应活性和成醛正/异比无明显下降. 该催化体系对不同长链烯烃氢甲酰化反应同样具有促进作用.
苏珂 , 蒋红斌 , 朱德明 , 付海燕 , 郑学丽 , 袁茂林 , 李瑞祥 , 陈华 . 十六烷基三羟乙基溴化铵促进1-辛烯氢甲酰化水/有机两相反应研究[J]. 化学学报, 2013 , 71(05) : 844 -848 . DOI: 10.6023/A13020164
A persisting problem in homogenous olefin hydroformylation is the recovery and reuse of the expensive rhodium catalyst. The aqueous/organic biphasic catalysis offers a feasible way to solve the problem of separation of products from catalysts. However, owing to very low solubility of higher olefin in the aqueous phase and the higher energy barrier of olefin transfer from the organic phase to the aqueous phase, the reaction rate is extremely low. The addition of a surfactant is a practical way to accelerate long-chain alkene hydroformylation in aqueous/organic bisphasic solution. In this paper, the influence of cetyltrihydroxyethyl ammonium bromide (CTHAB) on 1-octene hydroformylation in aqueous/organic two-phase solution with HRh(CO)(TPPTS)3 as catalyst and TPPTS [P(m-C6H4SO3Na)3] as ligand was investigated. It was confirmed that a complex formed between CTHAB and HRh(CO)(TPPTS)2 and the complex was further led to the enrichment of the catalytically active species on the micelle surface. Compared with the traditional surfactant cetyltrimethyl ammonium bromide (CTAB), results showed that the addition of surfactant CTHAB not only accelerated the 1-octene hydroformylation but also improved the molar ratio of linear to branched aldehydes. The effects of various reaction parameters, such as concentration of surfactants, [TPPTS]/[Rh] molar ratio, pressure of syngas and recycling number of catalytic system were investigated. Under the mild conditions: [Rh]=0.8 mmol/L, [TPPTS]/[Rh]=40, [CTHAB]=4.0 mmol/L, 90 ℃, 0.5 MPa, 1.5 h, the TOF and the molar ratio of linear to branched aldehydes reached up to 497 h-1 and 25.6, respectively. The catalyst-containing aqueous could be easily separated from organic phase and efficiently reused for seven times without evident loss of activity and regioselectivity. Meanwhile, the hydroformylation of different chain length olefins was also promoted by CTHAB.

/
| 〈 |
|
〉 |