

蜂窝状CuO和Fe2O3纳米管协同光电还原CO2
收稿日期: 2012-12-20
网络出版日期: 2013-04-17
基金资助
项目受国家青年自然科学基金(No. 21203114)、山东省优秀中青年科学家科研奖励基金(No. BS2012NJ008)和山东京博控股股份有限公司资助.
Honeycomb Cupric Oxide and Hematite Nanotubes Synergism Photoelectro-reduction CO2
Received date: 2012-12-20
Online published: 2013-04-17
Supported by
Project supported by the National Natural Science Foundation of China (Grant No. 21203114), Promotive Research Fund for Excellent Young and Middle-aged Scientists of Shandong Province (Grant No. BS2012NJ008) and Shandong Jingbo Holdings Company Limited.
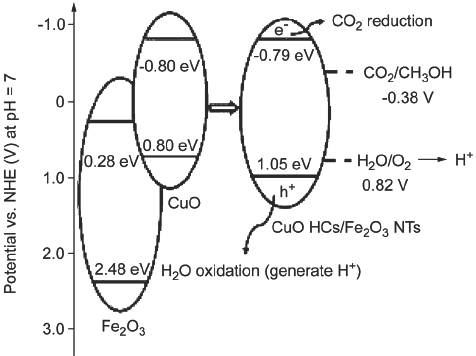
采用阳极氧化法在Fe上原位生长直立有序的Fe2O3纳米管阵列, 脉冲电沉积法将蜂窝状CuO组装到Fe2O3 NTs上, 得到CuO HCs/Fe2O3 NTs催化剂. 结果表明: Fe2O3 NTs呈“火山状”规则生长, 蜂窝状CuO均匀分布在Fe2O3 NTs表面. 修饰后, 材料的能隙由原来的2.03 eV窄化为1.84 eV. 同时发现该催化剂具有优良的光催化还原性能和电催化还原性能, 光电催化还原CO2的主要生成产物为甲醇, 甲醇在6 h时含量峰值为3.77 mmol/L. 该研究对光电催化还原CO2有一定的指导和借鉴意义.
王祜英 , 徐金凤 , 井华 , 张军 , 李培强 , 路福绥 . 蜂窝状CuO和Fe2O3纳米管协同光电还原CO2[J]. 化学学报, 2013 , 71(06) : 941 -946 . DOI: 10.6023/A12121073
Vertically aligned Fe2O3 nanotubes (Fe2O3 NTs) arrays were prepared on the iron foil by potentiostatic anodization method. The anodization experiments were performed under 30 V for 2 h (20 ℃). The ferrous foil was working electrode and titanium foil was counter electrode. The electrolytes were consisted of 0.25 wt% NH4F in an aqueous solution (Volume of water:glycol=3:97). After sonicating for 5 min, the samples were put under oxygen atmosphere with the flow rate of 60 sccm (standard cubic centimeters/min) and heated to 500 ℃ with the rate of 3 ℃•min-1 and maintained at 500 ℃ for 2 h. Then cooled to room temperature with the rate of 3 ℃•min-1. Honeycomb structure cupric oxide (CuO HCs) were decorated on the surface of Fe2O3 NTs by the pulse electrochemical deposition method. This was done by CHI660D potentiostat in 1 mol•L-1 CuSO4 solution (40 ℃) in a three-electrode configuration. The Fe2O3 NTs electrode worked as counter electrode, Hg/Hg2Cl2 in saturated KCl solution as reference electrode, and platinum wire as working electrode, respectively. A 10 ms cathodic pulse with a current density of -70 mA•cm-2 was employed. After that followed by a 1 ms anodic pulse and its density was +70 mA•cm-2 to prevent damage of the Fe2O3 NTs structure. Finally was a 1000 ms rest time. The electrodeposition time was 36 s, with a forward duty cycle of 0.99% (considering cathodic, anodic and rest components). Then the samples were annealed under oxygen atmosphere with the flow rate of 60 sccm then heated to 300 ℃ with the rate of 3 ℃•min-1 and maintained at 300 ℃ for 2 h. Then cooled to room temperature with the rate of 3 ℃•min-1. Then the CuO HCs/Fe2O3 NTs catalysis was obtained. The SEM images showed that CuO HCs distributed uniformly on the surface of Fe2O3 NTs that arranged in order were volcano-like. Measured by UV-vis DRS (ultraviolet-visible diffuse reflection spectrum) and valence band photoemission spectrum, its band energy has been narrowed from 2.03 eV to 1.84 eV after CuO decorating. It also has admirable photocatalytic and electrocatalytic reduction performance. The main product of photoelectrocatalytic CO2 reduction identified by chromatography was methanol and its concentration reached 3.77 mmol/L after 6 h. The study has some guidance and reference significance for photoelectrocatalytic CO2 reduction.

Key words: honeycomb CuO; Fe2O3 NTs; photoelectrocatalytic; CO2 reduction; methanol
/
| 〈 |
|
〉 |