

全固态锂离子电池的研究及产业化前景
All-solid-state Lithium Ion Battery: Research and Industrial Prospects
Received date: 2013-02-02
Online published: 2013-04-17
Supported by
Project supported by the National Natural Science Foundation of China (No. 51274239).
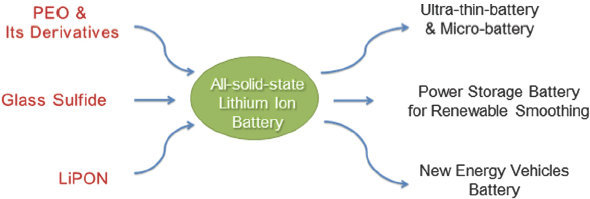
全固态锂离子电池具有安全性能高、能量密度大、工作温度区间广等优点, 是锂离子电池领域的研究热点. 固体电解质的开发是全固态锂离子电池实现应用的先决条件, 目前国内外研究比较广泛、应用前景较好的固体电解质主要有聚氧乙烯及其衍生物体系的聚合物电解质、LiPON薄膜电解质以及玻璃态硫化物体系的无机电解质三种. 近两年,在固体电解质的研究已取得很大进展的基础上, 人们正在将研究重点转向全固态电池结构设计及生产技术上, 并不断有样品电池面世. 本文从固体电解质的发展历史、最新研究进展、电池生产技术以及产业化应用前景这几个方面, 分别对以上三种体系的电解质及其电池进行综述, 以探索全固态锂离子电池的商品化前景.
刘晋 , 徐俊毅 , 林月 , 李劼 , 赖延清 , 袁长福 , 张锦 , 朱凯 . 全固态锂离子电池的研究及产业化前景[J]. 化学学报, 2013 , 71(06) : 869 -878 . DOI: 10.6023/A13020170
All-solid-state lithium ion battery has become an important focus due to higher safety, higher energy density and wider operating temperature compared to the commercial lithium ion battery with liquid organic electrolyte. Research and development of solid electrolyte are the keys for the successful market penetration of all-solid-state lithium ion battery. Nowadays, three kinds of solid electrolytes, polyethylene-oxide (PEO) as well as its derivatives based polymer electrolyte, LiPON thin film electrolyte, and glassy sulfide electrolyte, are widely studied and open very interesting new application prospects of all-solid-state lithium ion battery. Three major parameters of ionic conductivity, compatibility with electrodes, and manufacturing costs are used to evaluate the application prospects of the electrolyte. Based on that, PEO and its derivatives have low fabricating cost and good compatibility with electrodes. However, because of low lithium ionic conductivity at ambient temperature, the batteries using this electrolyte needs to work at high temperatures with a temperature control system. LiPON is most suitable for ultra-thin-battery and micro-battery, which present long cycle life and good rate performance. But, it is difficult for large-scale production of the batteries due to high cost and complex manufacturing processes. Glassy sulfide electrolyte exhibits the highest lithium ion conductivity (10-3~10-2 S/cm at 25 ℃) among the three electrolytes, which is close to the level of liquid organic electrolyte and meet the requirement in industrial application. However, advanced manufacturing technologies of the battery are required for the improvement of contacts at electrolyte/electrodes interface. In recent years, all-solid-state battery samples and pilot production lines are available on the market. In this review, we summarize the research progresses and production technologies of batteries based on the three solid electrolytes, and attempt to explore the commercial applications of all-solid-state lithium ion battery.

[1] Quartarone, E.; Mustarelli, P. Chem. Soc. Rev. 2011, 40, 2525.
[2] Cai, Y.; Li, Z.-J.; Zhang, H.-L.; Fan, X.; Zhang, S.-J. Acta Chim. Sinica 2010, 68, 1017. (蔡燕, 李在均, 张海朗, 范旭, 张锁江, 化学学报, 2010, 68, 1017.)
[3] Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J. P.; Phan, T. N.; Bertin, D.; Gigmes, D.; Devaux, D.; Denoyel, R.; Armand, M. Nat. Mater. 2013, DOI: 10. 1038/NMAT 3602.
[4] Zhang, J.-B.; Lian, F.; Gao, X.-P.; Li, J.-G.; Fan, L.-Z.; He, X.-M. Sci. Sin. Chim. 2012, 42, 1252. (张建波, 连芳, 高学平, 李建刚, 范丽珍, 何向明, 中国科学: 化学, 2012, 42, 1252.)
[5] Xi, J.-Y.; Ma, X.-M.; Cui, M.-Z.; Tang, X.-Z. Acta Chim. Sinica 2005, 63, 401. (席靖宇, 马晓梅, 崔梦忠, 唐小真, 化学学报, 2005, 63, 401.)
[6] Ogawa, M.; Yoshida, K.; Harada, K. SEI Tech. Rev. 2012, 74, 88.
[7] Kang, Y.; Lee, W.; Hack, Suh D.; Lee, C. J. Power Sources 2003, 119~121, 448.
[8] Fenton, D. E.; Parker, J. M.; Wright, P. V. Polymer 1973, 14, 589.
[9] Feuillade, G.; Perche, P. J. Appl. Electrochem. 1975, 5, 63.
[10] Armand, M. B.; Chavagno, J. B.; Dulot, M. J. Fast Ion Transport in Solids-electrode and Electrolytes Conference, North Holland Publishers Co., New York, 1979, pp. 131~134.
[11] Wright, P. V. Electrochim. Acta 1998, 43, 1137.
[12] Zhao, F.; Qian, X.-M.; Wang, E.-K.; Dong, S.-J. Prog. Chem. 2002, 14, 374. (赵峰, 钱新明, 汪尔康, 董绍俊, 化学进展, 2002, 14, 374.)
[13] Fullerton-Shirey, S. K.; Maranas, J. K. Macromolecules 2009, 42, 2142.
[14] Edman, L.; Ferry, A.; Doeff, M. M. J. Mater. Res. 2000, 15, 1950.
[15] He, D.; Cho, S. Y.; Kim, D. W.; Lee, C.; Kang, Y. Macromolecules 2012, 45, 7931.
[16] Ayd?n, H.; ?enel, M.; Erdemi, H.; Baykal, A.; Tülü, M.; Ata, A.; Bozkurt, A. J. Power Sources 2011, 196, 1425.
[17] Aihara, Y.; Kuratomi, J.; Bando, T.; Iguchi, T.; Yoshida, H.; Ono, T.; Kuwana, K. J. Power Sources 2003, 114, 96.
[18] Zhang, Z.; Jin, J.; Bautista, F.; Lyons, L.; Shariatzadeh, N.; Sherlock, D.; Amine, K.; West, R. Solid State Ionics 2004, 170, 233.
[19] Walkowiak, M.; Schroeder, G.; Gierczyk, B.; Waszak, D.; Osińska, M. Electrochem. Commun. 2007, 9, 1558.
[20] Krawiec, W.; Scanlon, J. L. G.; Fellner, J. P.; Vaia, R. A.; Vasudevan, S.; Giannelis, E. P. J. Power Sources 1995, 54, 310.
[21] Derrien, G.; Hassoun, J.; Sacchetti, S.; Panero, S. Solid State Ionics 2009, 180, 1267.
[22] Gu, D.-M.; Li, Y.-C.; Yang, L.; Xiao, Y. Acta Chim. Sinica 2010, 68, 2367. (顾大明, 李已才, 杨柳, 肖宇, 化学学报, 2010, 68, 2367.)
[23] Moreno, M.; Quijada, R.; Santa Ana, M. A.; Benavente, E.; Gomez-Romero, P.; González, G. Electrochim. Acta 2011, 58, 112.
[24] Do, N. S. T.; Schaetzl, D. M.; Dey, B.; Seabaugh, A. C.; Fullerton-Shirey, S. K. J. Phys. Chem. C 2012, 116, 21216.
[25] Przyluski, J.; Siekierski, M.; Wieczorek, W. Electrochim. Acta 1995, 40, 2101.
[26] Wu, H.; Cummings, O. T.; Wick, C. D. J. Phys. Chem. B 2012, 116, 14922.
[27] Ibrahim, S.; Yasin, S. M. M.; Nee, N. M.; Ahmad, R.; Johan, M. R. J. Non-Cryst. Solids 2012, 358, 210.
[28] Croce, F.; Persi, L.; Scrosati, B.; Serraino-Fiory, F.; Plichta, E.; Hendrickson, M. A. Electrochim. Acta 2001, 46, 2457.
[29] Gu, N.-Y.; Ao, H.; Pei, J.-J. Chem. J. Chin. Univ. 2012, 33, 1295. (古宁宇, 敖鹤, 裴建军, 高等学校化学学报, 2012, 33, 1295.)
[30] Tang, C. Y.; Hackenberg, K.; Fu, Q.; Ajayan, P. M.; Ardebili, M. Nano Lett. 2012, 12, 1152.
[31] Kim, S. K.; Kim, D. G.; Lee, A.; Sohn, H. S.; Wei, J. J.; Nguyen, N. A.; Mackay, M. E.; Lee, J. C. Macromolecules 2012, 45, 9347.
[32] Chinnam, P. R.; Wunder, S. L. J. Mater. Chem. A 2013, 1, 1731.
[33] Gozdz, A. S.; Scumutz, C. N.; Tarascon, J. M. US 5296318, 1994 [Chem. Abstr. 1994, 121, 413962].
[34] Zhu, Y.-M.; Ren, X.-F.; Li, N. Chemistry 2010, 12, 1073. (朱永明, 任雪峰, 李宁, 化学通报, 2010, 12, 1073.)
[35] Bates, J. B.; Dudney, N. J.; Gruzalski, R. A.; Choudhury, A.; Luck, C. F. Solid State Ionics 1992, 53~56, 647
[36] Hong, T. K.; Taehong, M.; Chinho, P.; Sang, W. J.; Ho, Y. P. J. Power Sources 2013, doi: 10.1016/j.jpowersour.2012.12.109.
[37] Suzuki, N.; Inaba, T.; Shiga, T. Thin Solid Films 2012, 520, 1821.
[38] Barrau, B.; Ribes, M.; Maurin, M. J. Non-Cryst. Solids 1980, 37, 1.
[39] Wada, H.; Menetrier, M.; Levasseur, A.; Hagenmuller, P. Mater. Res. Bull. 1983, 18, 189.
[40] Aotani, N.; Iwamoto, K.; Takada, K.; Kondo, S. Solid State Ionics 1994, 68, 35.
[41] Hirai, K.; Tatsumisago, M.; Minami, T. Solid State Ionics 1995, 78, 269.
[42] Mizuno, F.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. Solid State Ionics 2006, 177, 2721.
[43] Kanno, R.; Hata, T.; Kawamoto, Y.; Irie, M. Solid State Ionics 2000, 130, 97.
[44] Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; Mitsui, A. Nat. Mater. 2011, 10, 682.
[45] Wang, F. M.; Hu, C. C.; Lo, S. C.; Wang, Y. Y.; Wan, C. C. Solid State Ionics 2009, 180, 405.
[46] Zhang, J. W.; Huang, X. B.; Wei, H.; Fu, J. W.; Huang, Y. W.; Tang, X. Z. J. Solid State Electrochem. 2012, 16, 101.
[47] Hayashia, A.; Minamia, K.; Ujiieb, S.; Tatsumisago, M. J. Non-Cryst. Solids 2010, 356, 2670.
[48] Hassoun, J.; Verrelli, R.; Reale, P.; Panero, S.; Mariotto, G.; Greenbaum, S.; Scrosati, B. J. Power Sources 2013, 229, 117.
[49] Hamon, Y.; Douard, A.; Sabary, F.; Marcel, C.; Vinatier, P.; Pecquenard, B.; Levaseur, A. Solid State Ionics 2006, 177, 257.
[50] Mascaraque, N.; Fierro, J. L. G.; Duran, A.; Munoz, F. Solid State Ionics 2013, 233, 73.
[51] Kobayashi, Y.; Seki, S.; Mita, Y.; Ohno, Y.; Miyashiro, H.; Charest, P.; Guerfi, A.; Zaghib, K. J. Power Sources 2008, 185, 542.
[52] Damen, L.; Hassoun, J.; Mastragostino, M.; Scrosati, B. J. Power Sources 2010, 195, 6902.
[53] Angulakshmi, N.; Nahm, K. S.; Nair, J. R.; Gerbaldi, C.; Bongiovanni, R.; Penazzi, N.; Stephan, A. M. Electrochim. Acta 2013, 90, 179.
[54] Oh, B.; Vissers, D.; Zhang, Z.; West, R.; Tsukamoto, H.; Amine, K. J. Power Sources 2003, 119~121, 442.
[55] Niitani, T.; Shimada, M.; Kawamura, K.; Dokko, K.; Rho, Y. H.; Kanamura, K. Electrochem. Solid-State Lett. 2005, 8, A385.
[56] Koo, M.; Park, K.; Lee, S. H.; Suh, M.; Jeon, D. Y.; Choi, J. W.; Kang, K.; Lee, K. J. Nano Lett. 2012, 12, 4810.
[57] Sagane, F.; Shimokawa, R.; Sano, H.; Sakaebe, H.; Iriyama, Y. J. Power Sources 2013, 225, 245.
[58] Akridge, J. R.; Vourlis, H. Solid State Ionics 1986, 18~19, 1082.
[59] Kim, J.; Eom, M.; Noh, S.; Shin, D. J. Power Sources 2012, doi: 10.1016/j.jpowersour.2012.12.049.
[60] Koji, K. Next-Gen Batteries Going All-Solid: Demand for Large Size Batteries in EVs and Stationary Use Driving Development. 2010, July 1, http://techon.nikkeibp.co.jp/article/HONSHI/20100628/ 183827/?P=5 (accessed Apr. 10, 2013).
[61] Kato, Y.; Kawamoto, K.; Kanno, R.; Hirayama, M. Electrochemistry 2012, 80, 749.
[62] Fish, J. S.; Li, C. P.; Fehribach, J. D.; Wolden, C. A.; Hayre, R. O.; Bunge, A. L.; Goodyer, C. E. Electrochim. Acta 2012, 83, 454.
[63] Takada, K.; Ohta, N.; Zhang, L. Q.; Fukuda, K.; Sakaguchi, I.; Ma, R. Z.; Osada, M.; Sasaki, T. Solid State Ionics 2008, 179, 1333.
[64] Ogawa, M.; Kanda, R.; Yoshida, K.; Uemura, T.; Harada, K. J. Power Sources 2012, 205, 487.
[65] Satoshi, O. Japanese Researchers Seeking to Print Out Li-polymer Battery, 2011, Jan 7, http://techon.nikkeibp.co.jp/english/NEWS_ EN/ 20100107/179028/ (accessed Jan. 20, 2013).
[66] Okubo, C. GS Caltex Exhibition of Thin Film All-solid-state Lithium ion Battery Used in Energy Harvesting, 2011, Mar, 8, http://china. nikkeibp.com.cn/news/econ/55518-20110307.html (accessed Apr. 6, 2013).
[67] Tsunenori, T. Idemitsu Showcases A6-size Laminated All-solid Li-ion Battery, 2010, Mar 5, http://techon.nikkeibp.co.jp/english/ NEWS_EN/20100305/180872/ (accessed Mar. 23, 2013).
[68] Kouji, K.; Hideyoshi, K.; Hiroki, Y. The First Step is New Materials to Boost Capacity, 2010, Feb 1, http://techon.nikkeibp.co.jp/article/ HONSHI/20100127/179674/?P=1 (accessed Mar. 19, 2013).
[69] Naoshige, S. Toyota Prototypes All-solid-state Battery With 5x Higher Output Density, 2012, Sept 26, http://techon.nikkeibp.co.jp/ english/NEWS_EN/20120926/241911/ (accessed Jan. 20, 2013).Katherine, B. Safer, Longer-Lasting Batteries for Cars, 2010, July 20, http://www.technologyreview.com/energy/25825/ (accessed Apr. 18, 2013).
/
| 〈 |
|
〉 |