

单核Gd化合物的场依赖磁弛豫和磁热效应研究
收稿日期: 2013-03-21
网络出版日期: 2013-05-06
基金资助
项目受国家自然科学基金(No. 90922033)和国家重点基础研究发展计划(Nos. 2010CB934601, 2013CB933400)资助.
Field-Dependent Magnetic Relaxation and Magnetocaloric Effect in Mononuclear Gd Complexes
Received date: 2013-03-21
Online published: 2013-05-06
Supported by
Project supported by the National Natural Science Foundation of China (No. 90922033) and the National Basic Research Program of China (Nos. 2010CB934601, 2013CB933400).
钱康 , 王炳武 , 王哲明 , 苏刚 , 高松 . 单核Gd化合物的场依赖磁弛豫和磁热效应研究[J]. 化学学报, 2013 , 71(07) : 1022 -1028 . DOI: 10.6023/A13030319
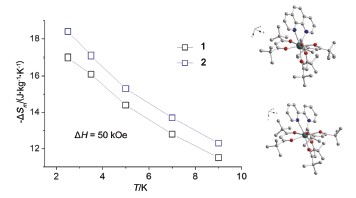
Two new mononuclear Gd complexes Gd(THD)3L (THD=2,2,6,6-tetramethylheptane-3,5-dione, L=Phen (1), Bpy (2)) based on b-diketone type ligand with Phen and Bpy as capping ligands were synthesized, respectively. To obtain the complexes, the THD liangd (3 mmol) and capping ligand (1 mmol) were mixed and dissolved in ethanol (10 mL), followed by adding of 5 mL aqueous solution of KOH (3 mmol) and slowly heating to remove the α-H of THD ligand. And then 5 mL aqueous solution of Gd(NO3)3 was added dropwise. The white precipitate was collected and recrystallized in the mixture of ethanol and acetone to get colorless transparent crystals. Their structures were determined by the single-crystal X-ray diffraction method, found crystallized in P-1 space group, and gave an eight-coordinated coordination environment of Gd3-with D4d local symmetry. Magnetic susceptibility measurements of powder samples were carried out by SQUID magnetometer at 1000 Oe dc field from 2 to 300 K, and the χmT value at room temperatures were 7.89 and 8.25 cm3·K·mol-1, respectively, in good agreement with the expected value. The magnetization measurements reveal that the magnetic entropy changes (-ΔSm) at 2.5 K from 0 to 5 Tesla magnetic field are as large as 17.0 and 18.4 J·kg-1·K-1 for 1 and 2, respectively. The ac susceptibility shows an obvious slow magnetic relaxation phenomenon. By analyzing the temperature and magnetic field dependences of the relaxation time, we found that the resonant phonon trapping mechanism is predominant when the field is lower than 1 T and the direct process plays a role when the field is larger than 2 T.
Key words: Gd; field-dependent relaxation; magnetocaloric effect
/
| 〈 |
|
〉 |