

芳基亚胺-脒基稀土烷基化合物的合成及其催化异戊二烯聚合研究
Synthesis and Catalytic Isoprene Polymerization of Rare-Earth Alkyl Complexes Supported by An Arylimine-amidinate Ligand
Received date: 2013-04-22
Online published: 2013-05-24
Supported by
Project supported by 973 program (Grant No. 2012CB821600).
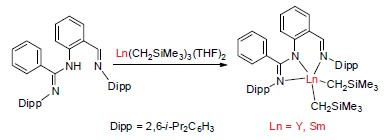
制备了芳基亚胺-脒基稀土二烷基化合物[NNN]Ln(CH2SiMe3)2 {[NNN]=[2-C(H)NDippC6H3NHC(Ph)NDipp], Dipp=2,6-i-Pr2C6H3, Ln=Y (2), Sm (3)}, 2和3通过了1H NMR, 13C NMR, IR和元素分析测试, 通过X-ray确定了化合物2的晶体结构. 加入[Ph3C][B(C6F5)4]和烷基铝, 两个化合物均能高效催化异戊二烯聚合, 具有较好的3,4-选择性(88%), 中等的立体选择性(rr=50%), 较高的分子量(Mn=6.8×104)和较窄的分子量分布(Mw/Mn=1.15). 同时发现, 烷基铝和[Ph3C][B(C6F5)4]的比例影响催化聚合的区域选择性.
高东静 , 胡泓梵 , 崔春明 . 芳基亚胺-脒基稀土烷基化合物的合成及其催化异戊二烯聚合研究[J]. 化学学报, 2013 , 71(08) : 1125 -1128 . DOI: 10.6023/A13040433
Reaction of the arylimine-amidinate ligand[NNN]H (1) ([NNN]=[2-C(H)NDippC6H3NHC(Ph)NDipp], Dipp=2,6-i-Pr2C6H3) with Ln(CH2SiMe3)3(THF)2 (Ln=Y, Sm) in n-hexane at -78℃ followed by stirring the mixture for 3 h at room temperature afforded[NNN]Ln(CH2SiMe3)2 (Ln=Y, 2; Ln=Sm, 3) in 75% and 50% yields after work up. 2 and 3 have been characterized by 1H NMR, 13C NMR, IR spectroscopy, elemental analysis. The molecular structure of 2 has been confirmed by X-ray analysis. Complexes 2 and 3 catalyzed the polymerization of isoprene in the presence of[Ph3C][B(C6F5)4] (1 equiv.) and AlEt3 (10 equiv.) at 25℃. 2 converted 250 equivalents of isoprene into polyisoprene with a narrow molecular weight distribution (Mw/Mn=1.20) and a 3,4-rich microstructure (3,4-selectivity: 78%, rr=50%) in 12 h. A significant increase of 3,4-selectivity was observed in the presence of one equivalent of AlEt3 (3,4-selectivity: 87%). When i-Bu2AlH (10 equiv.) was used instead of AlEt3, the catalytic activity and selectivity did not change noticeably while the resulting polyisoprenes feature a broad molecular weight distribution (Mw/Mn=2.45). When the amounts of i-Bu2AlH decreased from 5 to 1 equivalent, a significant increase of 3,4-selectivity and number-average molecular weight have been observed while the molecular distributions became small. The catalyst is active within a wide range of temperatures from low temperature to 40℃. At 40℃, a similar selectivity (3,4-selectivity: 78%, Mw/Mn=1.43) to that found at 25℃ but the increased reaction rate has been observed. High stereo- and regioselectivity and narrow molecular weight distributions were obtained at -20℃ (3,4-selectivity: 88%, rr=52%, Mw/Mn=1.15). The samarium alkyl 3 exhibited a high activity but low selectivity (25℃, 3 h, 3,4-selectivity: 67%, Mw/Mn=1.71) compared to the yttrium alkyl 2.

[2] Chen W.-Q.; Wang F.-S. Sci China Ser B-Chem, 2009, 39, 1006 (in chinese).
(陈文启, 王佛松, 中国科学B辑:化学, 2009, 39, 1006.)
[3] Zimmermann, M.; Anwander, R. Chem. Rev. 2010, 110, 6194.
[5] Arndt, S.; Beckerle, K.; Zeimentz, P. M.; Spaniol, T. P.; Okuda, J. Angew. Chem. Int. Ed. 2005, 44, 7473.
[6] Zhang, L. X.; Luo, Y.; Hou, Z. J. Am. Chem. Soc. 2005, 127, 14562.
[8] (a) Wang, B. L.; Cui, D. M.; Lv, K. Macromolecules. 2008, 41, 1983; (b) Li, S.; Cui, D.; Li, D.; Hou, Z. Organometallics. 2009, 28, 4814; (c) Döring, C.; Kretschmer, W. P.; Bauer, T.; Kempe, R. Eur. J. Inorg. Chem. 2009, 2009, 4255; (d) Du, G.; Wei, Y.; Ai, L.; Chen, Y.; Xu, Q.; Liu, X.; Zhang, S.; Hou, Z.; Li, X. Organometallics. 2011, 30, 160.
[9] Tobisch, S. J. Mol. Struc-theochem, 2006, 771, 171.
[10] (a) Wolpers, J. EP 456902 A1, 1991 [Chem. Abstr. 1991, 116, 61376]; (b) Massie, J. D. US 5356997, 1994 [Chem. Abstr. 1994, 122, 57979].
[11] Hu, H.; Cui, C. Organometallics. 2012, 31, 1208.
[12] (a) Boeré, R. T.; Klassen, V.; Wolmershäuser, G. J. Chem. Soc., Dalton Trans. 1998, 4147; (b) Hultzsch, K. C.; Spaniol, H. P.; Okuda, J. Angew. Chem. Int. Ed. 1999, 38, 227.
[13] (a) Bambirra, S.; Bouwkamp, M. W.; Meetsma, A.; Hessen, B. J. Am. Chem. Soc. 2004, 126, 9182; (b) Bambirra, S.; Otten, E.; Leusen, D. V.; Meetsma, A.; Hessen, B. Z. Anorg. Allg. Chem. 2006, 632, 1950; (c) Li, D.; Li, S.; Cui, D.; Zhang, X. Organometallics. 2010, 29, 2186.
[14] Schumann, H.; Freckmann, D. M. M.; Dechert, S. Z. Anorg. Allg. Chem. 2002, 628, 2422.
[15] Zhang, L. X.; Nishiura, M.; Yuki, M.; Luo, Y.; Hou, Z. M. Angew. Chem. Int. Ed. 2008, 47, 2642.CCDC- 940264 contains the supplementary crystallographic data for compound 2. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
/
| 〈 |
|
〉 |