

锂空气电池非水基电解液的优化与研究进展
收稿日期: 2013-04-10
网络出版日期: 2013-06-27
基金资助
项目受哈尔滨市优秀学科带头人专项资金(No. 2012RFXXG99)资助.
Research Progress and Optimization of Non-aqueous Electrolyte for Lithium Air Batteries
Received date: 2013-04-10
Online published: 2013-06-27
Supported by
Project supported by the Outstanding Subject Leaders Special Foundation of Harbin, China (No. 2012RFXXG99).
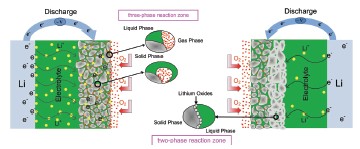
锂空气电池是介于燃料电池和锂电池之间的一种新一代高性能绿色二次电池, 其理论比能量高达11140 Wh/kg (Li), 是锂离子电池的6~9倍, 同时具有输出电压稳定、环境友好等优点, 应用前景广阔. 电解液是电池中重要的组成部分, 在决定电池的电化学性能方面起着至关重要的作用. 综述了锂空气电池中有机电解液、离子液体和固态电解质等三种非水基电解质的研究进展, 系统阐述了各电解液不同化学性质(电化学稳定性、离子导电率、极性)、物理性质(如介电常数、黏度、氧气溶解度、吸湿性)和物理化学性质(对阴极材料的浸润能力等)对锂空气电池放电比容量、大电流放电能力和循环性能的影响, 并对其未来的发展方向进行了展望.
顾大明 , 王余 , 顾硕 , 张传明 , 杨丹丹 . 锂空气电池非水基电解液的优化与研究进展[J]. 化学学报, 2013 , 71(10) : 1354 -1364 . DOI: 10.6023/A13040389
Lithium air battery is an advanced electrochemical power source that lies between fuel cell and lithium battery, and it is considered to be one of the most promising next generation batteries due to the fact that it may provide extremely high theoretical energy, i.e. 11140 Wh/kg (Li), which is estimated to be 6~9 times higher than that of the lithium ion batteries. The reason for such high theoretical energy is the use of Li sheet (with extremely high energy density) as anode electrode and O2 as cathodic reactant from the air. Other advantages of the battery include stable output voltage, cost effectiveness, and pollution free, and have broad application prospects. If it is successfully developed, the battery could be an excellent energy storage device for renewable energy sources such as wind, solar, and tidal energy, which brings a prospect for human to solve the problem of environment pollution and energy crisis. Electrolyte is a crucial component of lithium air battery and the electrochemical performance of the battery is determined by electrolyte to a great extent. Due to the react violently between lithium and water, it is not practical for lithium air battery to use directly an aqueous electrolyte unless the anode can be protected from degradation. In this paper, the authors presented the latest research progress on three kind of non-aqueous electrolyte, i.e. organic electrolyte, ionic liquid and solid electrolyte. We elaborated the influence of various chemical properties (electrochemical stability, conductivity, polarity), physical properties (dielectric constant, viscosity, oxygen solubility, hygroscopicity and electrolyte filling) and physicochemical properties (infiltration ability to cathode materials) on lithium air battery's performance, i.e. specific capacity, rate capacity and cycling efficiency. In addition, we also provided insights into the prospect of non-aqueous electrolyte for lithium air battery.

/
| 〈 |
|
〉 |