

设计合成nia拓扑的金属有机骨架化合物与CO2吸附
收稿日期: 2013-07-04
网络出版日期: 2013-09-06
基金资助
项目受国家自然科学基金(No. 20831002)资助.
Design and Synthesis of a Metal-organic Framework with nia Topology
Received date: 2013-07-04
Online published: 2013-09-06
Supported by
Project supported by the National Natural Science Foundation of China (No. 20831002).
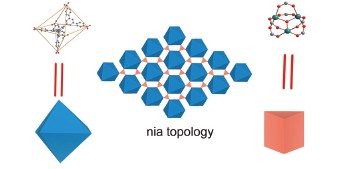
Nia拓扑结构是由两种分别是八面体和三棱柱构型的六连接节点连接形成的三维结构. 为了得到具有nia拓扑的金属有机骨架, 可以通过连接具有八面体构型的配体和具有三棱柱构型的金属簇实现. 常见的三棱柱构型的金属簇为M3O (M=金属, M三价)或者M3OH(M二价). 本文通过设计合成的枝状的具有八面体构型的有机配体1,3,5-tris(3,5-di-(4-carboxy-phenyl-1-yl)phenyl-1-yl)benzene (TDCPB), 在溶剂热条件下, 利用硝酸镍合成了一种新型的具有nia拓扑结构的金属有机骨架化合物JUC-105. 通过X射线单晶衍射表征, 该金属有机骨架化合物是由六棱柱的Ni3OH金属簇与八面体的TDCPB共同构筑的三维骨架结构, 具有较大的孔道(1.1 nm). 通过粉末衍射以及热重分析, 研究了这种金属有机骨架的结构稳定性. 气体吸附数据表明, 这种骨架没有氮气吸附能力, 但是表现了一定的CO2吸附能力.
贾江涛 , 王蕾 , 赵晴 , 孙福兴 , 朱广山 . 设计合成nia拓扑的金属有机骨架化合物与CO2吸附[J]. 化学学报, 2013 , 71(11) : 1492 -1495 . DOI: 10.6023/A13070702
Nia topology is 3D structure which is constructed by octahedral and trigonal prism building blocks. In order to obtain Metal-organic Framework (MOFs) with nia topology, we can connect ligands with octahedral configuration and metal clusters with trigonal prism configuration. The typical metal clusters with trigonal prism configuration are M3O (M=metal, M is trivalent) or M3OH (M is divalent). In this article, a dendritic ligand 1,3,5-tris(3,5-di-(4-carboxy-phenyl-1-yl)phenyl-1-yl)benzene (TDCPB) with ten phenyl rings and an octahedral configuration is designed and synthesized. We used the metal salt of NiCl2 and the octahedral ligand of TDCPB to construct a MOF (JUC-105) with nia topology in solvent thermal condition. (Details: In a 20 mL reactor, NiCl2·6H2O (30 mg) and the dendritic ligand TDCPB (10 mg) are added to a solvent of Dimethylacetamide (DMA, 3 mL), and then 3 drops of HBF4 solution are added. After ultrasonic diffusion for 2 min, it is heated at 100 ℃ for 72 h to obtain green single crystals) Single crystal diffraction is used to characterize its structure. JUC-105 crystallizes in P-62c space group. JUC-105 is constructed by Ni3OH cluster and TDCPB with a 3D structure. The dimension of the large channel is about 1.1 nm. X-ray diffraction and thermogravimetric analysis are used to analyse its thermal stability. The gas uptake of JUC-105 is also tested. It is found that JUC-105 does not have N2 adsorption. It may be because the weak stability of the structure after the guest molecular removed. However, this MOF has a CO2 uptake of 24 mL/g at 273 K, 1 atm. After the collapsing of the structure, windows of the pores are blocked and the dimension of the pore is much smaller. Therefore, the N2 molecules can not be adsorbed. Because the CO2 have smaller dimension than N2 molecules, and it can pass through the small windows, the structure still have a comparative CO2 adsorption.

Key words: Metal-organic Framework (MOF); nia topology; CO2 adsorption; self-assembly
[1] (a) Chae, H. K.; Eddaoudi, M.; Kim, J.; Hauck, S. I.; Hartwig, J. F.; O'Keeffe, M.; Yaghi, O. M. J. Am. Chem. Soc. 2001, 123, 11482; (b) Reinsch, H.; Marszalek, B.; Wack, J.; Senker, J.; Gil, B.; Stock, N. Chem. Commun. 2012, 48, 9486.
[2] (a) Tanaka, K.; Muraoka, T.; Hirayama, D.; Ohnish, A. Chem. Commun. 2012, 48, 8577; (b) Zhang, X.-F.; An, X.-H.; Liu, D.-H.; Yang, Q.-Y.; Yang, Z.-H.; Zhong, C.-L.; Lu, X.-H. Acta Chim. Sinica 2011, 69, 84. (张秀芳, 安晓辉, 刘大欢, 阳庆元, 杨祝红, 仲崇立, 陆小华, 化学学报, 2011, 69, 84.); (c) Wu, X.-J.; Yang, X.; Song, J.; Cai, W.-Q. Acta Chim. Sinica 2012, 70, 2518. (吴选军, 杨旭, 宋杰, 蔡卫权, 化学学报, 2012, 70, 2518.) (d) Zhou, Z.-E.; Xue, C.-Y.; Yang, Q.-Y.; Zhong, C.-L. Acta Chim. Sinica 2009, 67, 477. (周子娥, 薛春瑜, 阳庆元, 仲崇立, 化学学报, 2009, 67, 477.)
[3] Song, F.; Wang, C.; Falkowski, J. M.; Ma, L.; Lin, W. J. Am. Chem. Soc. 2010, 132, 15390.
[4] Chen, B.; Wang, L.; Zapata, F.; Qian, G.; Lobkovsky, E. B. J. Am. Chem. Soc. 2008, 130, 6718.
[5] Taylor-Pashow, K. M.; Della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. J. Am. Chem. Soc. 2009, 131, 14261.
[6] Tranchemontagne, D. J.; Mendoza-Cortes, J. L.; O'Keeffe, M.; Yaghi, O. M. Chem. Soc. Rev. 2009, 38, 1257.
[7] Yaghi, O. M.; O'Keeffe, M.; Ockwig, N. W.; Chae, H. K.; Eddaoudi, M.; Kim, J. Nature 2003, 423, 705.
[8] Delgado-Friedrichs, O.; O'Keeffe, M.; Yaghi, O. M. Acta Crystallogr. A 2006, 62, 350.
[9] Wang, Z.; Zhang, X.; Batten, S. R.; Kurmoo, M.; Gao, S. Inorg. Chem. 2007, 46, 8439.
[10] Chae, H. K.; Eddaoudi, M.; Kim, J.; Hauck, S. I.; Hartwig, J. F.; O'Keeffe, M.; Yaghi, O. M. J. Am. Chem. Soc. 2001, 123, 11482.
[11] Bai, Y. L.; Tao, J.; Huang, R. B.; Zheng, L. S.; Zheng, S. L.; Oshida, K.; Einaga, Y. Chem. Commun. 2008, 1753.
[12] Jia, J.; Sun, F.; Fang, Q.; Liang, X.; Cai, K.; Bian, Z.; Zhao, H.; Gao, L.; Zhu, G. Chem. Commun. 2011, 47, 9167.
[13] Jia, J.; Sun, F.; Borjigin, T.; Ren, H.; Zhang, T.; Bian, Z.; Gao, L.; Zhu, G. Chem. Commun. 2012, 48, 6010.
[14] Jia, J.; Lin, X.; Wilson, C.; Blake, A. J.; Champness, N. R.; Hubberstey, P.; Walker, G.; Cussen, E. J.; Schroder, M. Chem. Commun. 2007, 840.
/
| 〈 |
|
〉 |