

α,β-不饱和酸多相不对称氢化反应中的钯粒径和载体酸性效应
收稿日期: 2013-07-12
网络出版日期: 2013-09-13
基金资助
项目受国家自然科学基金(No. 20921092)资助.
Enantioselective Hydrogenation of α,β-Unsaturated Carboxylic Acids:Effects of Palladium Particle Size and Support Acidic Property
Received date: 2013-07-12
Online published: 2013-09-13
Supported by
Project supported by the National Natural Science Foundation of China (No. 20921092).
陈春辉 , 展恩胜 , 李勇 , 申文杰 . α,β-不饱和酸多相不对称氢化反应中的钯粒径和载体酸性效应[J]. 化学学报, 2013 , 71(11) : 1505 -1510 . DOI: 10.6023/A13070728
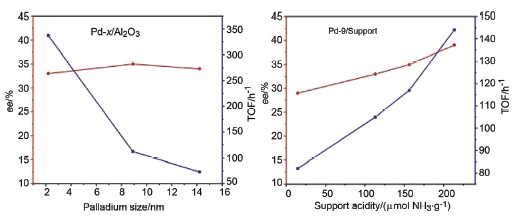
Effects of Pd particle size and support acidity on enantioselective hydrogenation of α,β-unsaturated carboxylic acids were systematically studied using Pd nanoparticles with different size immobilized on various oxides. Small Pd particles showed higher activity in the hydrogenation of (E)-2-methyl-2-pentenoic acid due to the larger fraction of edge sites which were more active in olefin hydrogenation; but they did not change the reaction mechanism and/or the adsorption mode of reaction intermediates. Similar correlations in the hydrogenation of (E)-2-methyl-2-butenoic acid and 2-acetamidoacrylic acid further confirmed that the size of Pd particles only mediated the activity but did not alter the enantioselectivity. On the other hand, the activity and the enantioselectivity were strongly dependent on the acidity of the support. The TOF and the ee value followed the order TiO2 > γ-Al2O3 > SiO2 > CeO2, suggesting that the acidic support favored the adsorption of the reaction intermediates.
[1] Yu, Z. K.; Jin, W. W.; Jiang, Q. B. Angew. Chem., Int. Ed. 2012, 51, 6060.
[2] Xie, J. H.; Zhou, Q. L. Acta Chim. Sinica 2012, 70, 1427. (谢建华, 周其林, 化学学报, 2012, 70, 1472.)
[3] Ma, X.; Li, W. F.; Fan, W. Z.; Tao, X. M.; Li, X. M.; Yao, Y.; Zhu, L. F.; Chen, H. H.; Xie, X. M.; Zhang, Z. G. Chin. J. Org. Chem. 2012, 32, 1353. (马欣, 李万方, 范为正, 陶晓明, 李晓明, 姚莹, 诸吕锋, 陈厚和, 谢小敏, 张兆国, 有机化学, 2012, 32, 1353.)
[4] Chen, Q. A.; Ye, Z. S.; Duan, Y.; Zhou, Y. G. Chem. Soc. Rev. 2013, 42, 497
[5] Irfan, M.; Glasnov, T. N.; Kappe, C. O. Chemsuschem. 2011, 4, 300.
[6] Izumi, Y.; Imaida, M.; Fukawa, H.; Akabori, S. Bull. Chem. Soc. Jpn. 1963, 36, 21.
[7] Orito, Y.; Imai, S.; Niwa, S. J. Chem. Soc. Jpn. 1979, 1118.
[8] Xiong, W.; Huang, Y. L.; Ma, H. X.; Chen, H.; Li, Y. Z.; Li, L. L.; Cheng, P. M.; Li, X. J. Acta Chim. Sinica 2003, 61, 922. (熊伟, 黄裕林, 马红霞, 陈华, 黎耀忠, 李蕾蕾, 程溥明, 李贤均, 化学学报, 2003, 61, 922.)
[9] Xiong, W.; Huang, Y. Y.; Chen, H.; Li, X. J. Acta Chim. Sinica 2005, 63, 1927. (熊伟, 黄艳轶, 陈华, 李贤均, 化学学报, 2005, 63, 1927.)
[10] Mallat, T.; Orglmeister, E.; Baiker, A. Chem. Rev. 2007, 107, 4863.
[11] Jiang, H. Y.; Chen, H. Acta Chim. Sinica 2012, 70, 297. (蒋和雁, 陈华, 化学学报, 2012, 70, 297.)
[12] Nitta, Y. J. Syn. Org. Chem. Jpn. 2006, 64, 827.
[13] Tungler, A.; Sipos, E.; Hada, V. Curr. Org. Chem. 2006, 10, 1569.
[14] Fujihara, H.; Tamura, M. J. Am. Chem. Soc. 2003, 125, 15742.
[15] Jansat, S.; Gómez, M.; Philippot, K.; Muller, G.; Guiu, E.; Claver, C.; Castillón, S.; Chaudret, B. J. Am. Chem. Soc. 2004, 126, 1592.
[16] Sawai, K.; Tatumi, R.; Nakahodo, T.; Fujihara, H. Angew. Chem., Int. Ed. 2008, 47, 6917.
[17] Ranganath, K. V. S.; Kloesges, J.; Schäfer, A. H.; Glorius, F. Angew. Chem., Int. Ed. 2010, 49, 7786.
[18] Nitta, Y.; Watanabe, J.; Okuyama, T.; Sugimura, T. J. Catal. 2005, 236, 164.
[19] Szöllösi, G.; Hermán, B.; Felföldi, K.; Fülöp, F.; Bartók, M. Adv. Synth. Catal. 2008, 350, 2804.
[20] Szöllösi, G.; Hermán, B.; Szabados, E.; Fülöp, F.; Bartók, M. J. Mol. Catal. A: Chem. 2010, 333, 28.
[21] Kun, I.; Török, B.; Felföldi, K.; Bartók, M. Appl. Catal. A: Gen. 2000, 203, 71.
[22] Bisignani, R.; Franceschini, S.; Piccolo, O.; Vaccari, A. J. Mol. Catal. A: Chem. 2005, 232, 161.
[23] Casagrande, A.; Franceschini, S.; Lenarda, M.; Piccolo, O.; Vaccari, A. J. Mol. Catal. A: Chem. 2006, 246, 263.
[24] György, S.; Zsolt, M.; Mihály, B. React. Kinet. Catal. Lett. 2009, 96, 319.
[25] Makra, Z.; Szöllösi, G.; Bartók, M. Catal. Today 2012, 181, 56.
[26] Borszeky, K.; Mallat, T.; Baiker, A. Catal. Lett. 1999, 59, 95.
[27] Solladié-Cavallo, A.; Hoernel, F.; Schmitt, M.; Garin, F. J. Mol. Catal. A: Chem. 2003, 195, 181.
[28] Toebes, M. L.; van Dillen, J. A.; de Jong, Y. P. J. Mol. Catal. A: Chem. 2001, 173, 75.
[29] Nitta, Y.; Kubota, T.; Okamoto, Y. Bull. Chem. Soc. Jpn. 2001, 74, 2161.
[30] Nitta, Y.; Kubota, T.; Okamoto, Y. J. Mol. Catal. A: Chem. 2004, 212, 155.
[31] Kubota, T.; Kubota, H.; Kubota, T.; Moriyasu, E.; Uchida, T.; Nitta, Y.; Sugimura, T.; Okamoto, Y. Catal. Lett. 2009, 129, 387.
[32] Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S. E. Angew. Chem., Int. Ed. 2009, 48, 60.
[33] Xiao, C.; Ding, H.; Shen, C.; Yang, T.; Hui, C.; Gao, H. J. J. Phys. Chem. C 2009, 113, 13466.
[34] Jin, M.; Liu, H.; Zhang, H.; Xie, Z.; Liu, J.; Xia, Y. Nano Res. 2011, 4, 83.
[35] Murphy, C. J.; Sau, T. K.; Gole, A. M.; Orendorff, C. J.; Gao, J.; Gou, L.; Hunyadi, S. E.; Li, T. J. Phys. Chem. B 2005, 109, 13857.
[36] Ma, R.; Semagina, N. J. Phys. Chem. C 2010, 114, 15417.
[37] Crespo-Quesada, M.; Yarulin, A.; Jin, M.; Xia, Y.; Kiwi-Minsker, L. J. Am. Chem. Soc. 2011, 133, 12787.
[38] Belelli, P. G.; Ferullo, R. M.; Castellani, N. J. Surf. Sci. 2010, 604, 386.
[39] Bürgi, T.; Baiker, A. Acc. Chem. Res. 2004, 37, 909.
[40] Hoxha, F.; van Vegten, N.; Urakawa, A.; Krurneich, F.; Mallat, T.; Baiker, A. J. Catal. 2009, 261, 224.
/
| 〈 |
|
〉 |