

高分散镍-硼/纳米多孔铜非晶态合金电极上葡萄糖的电催化氧化
收稿日期: 2013-05-31
网络出版日期: 2013-11-03
基金资助
项目受福建省自然科学基金(No. 2010J01292)、福建省纳米材料重点实验室基金(No. NM10-04)、华侨大学高层次人才科研启动基金(No. 08BS205)和国家自然科学基金(No. 21103055)资助.
Electrocatalytic Oxidation of Glucose on Highly Dispersed Ni-B/Nanoporous Cu Amorphous Alloy Electrode
Received date: 2013-05-31
Online published: 2013-11-03
Supported by
Project supported by the Fujian Provincial Natural Science Foundation (No. 2010J01292), Fund of Fujian Provincial Key Laboratory of Nanomaterials (No. NM10-04), and Program for Excellent Talents in Huaqiao University, China (No. 08BS205), and the National Natural Science Foundation of China (No. 21103055).
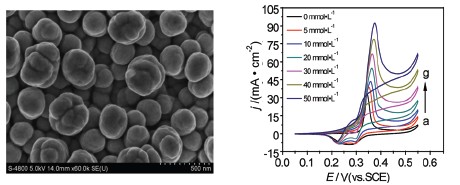
通过酸腐蚀去合金法处理热扩散制备的铜基表面铜锌合金得到纳米多孔铜(NPC)材料. 以NPC为载体,采用超声辅助化学镀制备Ni-B/NPC合金电极. X射线衍射(XRD)和扫描电镜(SEM)表明Ni-B/NPC电极呈现由高分散纳米颗粒组成的非晶态结构. 计时电流法(CA)结果表明化学镀5 min制备的Ni-B/NPC电极具有最大的电化学活性表面积(EASA). 循环伏安法(CV)结果表明,与块状镍电极相比,碱性介质中在Ni-B/NPC电极上葡萄糖起始氧化电位负移39 mV,氧化峰电流提高了18.9倍. 采用线性扫描伏安法(LSV)、CA和电化学阻抗谱(EIS)测定Ni-B/NPC电极对葡萄糖电催化氧化的电子转移系数(β)、电催化氧化反应速率常数(k)和葡萄糖的扩散系数(D)等动力学参数. 结果表明高分散Ni-B/NPC非晶态合金电极对碱性介质中葡萄糖的氧化具有较高的电催化活性和稳定性.
张树金 , 郑一雄 , 袁林珊 , 杨卫华 . 高分散镍-硼/纳米多孔铜非晶态合金电极上葡萄糖的电催化氧化[J]. 化学学报, 2013 , 71(12) : 1676 -1682 . DOI: 10.6023/A13050577
As glucose is the richest carbohydrate in nature and has extremely high energy density, direct glucose fuel cell (DGFC) is considered to be a promising power source. Catalyst is the key to the development of DGFC. The expensive noble metal catalysts hinder their commercial applications. Ni is a cheap metal with an excellent catalytic performance. Ni and its compounds using glassy carbon as the carrier have been developed for glucose oxidation, but the smooth surface of glassy carbon could not be conductive to dispersion of nanoparticles. The development of nanoporous materials may overcome the drawback. In this experiment, Cu-based surface Cu-Zn alloy was prepared by thermal diffusion and Nanoporous Copper (NPC) with high surface area was prepared by removing of Zn element from the alloy in 5% H2SO4 solution. NPC supported Nickel-Boron (Ni-B/NPC) alloy electrode was fabricated by ultrasonic-assisted electroless plating. The Ni-B/NPC alloy electrode was characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD) and electrochemical methods. XRD and SEM results showed Ni-B alloy electrode was amorphous structure with high dispersion of Ni-B nanoparticles. Chronoamperometry (CA) result found the prepared Ni-B/NPC amorphous alloy electrode with ultrasonic for 5 min had the highest electrochemical active surface area (EASA) and surface roughness. The CV results of glucose oxidation at the Ni-B/NPC amorphous alloy electrode showed the onset oxidation potential had a negative shift of 39 mV and the oxidation peak current density increased by 18.9 times compared with the bulk Ni electrode in alkaline media. Meanwhile, the kinetic parameters of electrocatalytic oxidation of glucose at the Ni-B/NPC amorphous alloy electrode were determined by linear sweep voltammetry (LSV), CA method and electrochemical impedance spectroscopy (EIS), such as charge transfer coefficient (β), catalytic rate constant (k) and diffusion coefficient (D). The results indicated the good electrocatalytic activity and stability of the highly dispersed Ni-B/NPC amorphous alloy electrode for glucose oxidation in alkaline medium, and the diffusion coefficient of glucose was improved by about two orders of magnitude relative to the reported value at the Ni-Cu/GC electrode. The Ni-B alloy should be a promising anodic catalyst for direct glucose fuel cell.

[1] Fujiwara, N.; Yamazaki, S. I.; Siroma, Z.; Ioroi, T.; Senoh, H.; Yasuda, K. Electrochem. Commun.2009, 11, 390.
[2] Basu, D.; Basu, S. Electrochim. Acta 2010, 55, 5775.
[3] Basu, D.; Basu, S. Electrochim. Acta 2011, 56, 6106.
[4] Park, S.; Boo, H.; Chung, T. D. Anal. Chim. Acta 2006, 556, 46.
[5] Yang, Y. J.; Hu, S. S. Electrochim. Acta 2010, 55, 3471.
[6] Xu, Q.; Yin, L. N.; Hou, C. T.; Liu, X. X.; Hu, X. Y. Sens. Actuators B 2012, 173, 716.
[7] Wang, Q. Y.; Cui, X. Q.; Chen, J. L.; Zheng, X. L.; Liu, C.; Xue, T. Y.; Wang, H. T.; Jin, Z.; Qiao, L.; Zheng, W. T. RSC Adv. 2012, 2, 6245.
[8] Xia, Q. F.; Luo, D.; Li, Z. J. Acta Chim. Sinica 2012, 70, 2079. (夏前芳, 罗丹, 李在均, 化学学报, 2012, 70, 2079.)
[9] Basu, D.; Basu, S. Int. J. Hydrogen Energy 2012, 37, 4678.
[10] Tao, B. R.; Miao, F. J.; Chu, P. K. Electrochim. Acta 2012, 65, 149.
[11] Basu, D.; Basu, S. Int. J. Hydrogen Energy 2011, 36, 14923.
[12] Yousef Elahi, M.; Heli, H.; Bathaie, S. Z.; Mousavi, M. F. J. Solid State Electrochem. 2007, 11, 273.
[13] Qiu, R.; Zhang, X. L.; Qiao, R.; Li, Y.; Kim, Y. I.; Kang, Y. S. Chem. Mater. 2007, 19, 4174.
[14] Jafarian, M.; Forouzandeh, F.; Danaee, I.; Gobal, F.; Mahjani, M. G. J. Solid State Electrochem. 2009, 13, 1171.
[15] Zheng, L.; Zhang, J. Q.; Song, J. F. Electrochim. Acta 2009, 54, 4559.
[16] Ganesh, V.; Farzana, S.; Berchmans, S. J. Power Sources 2011, 196, 9890.
[17] Chekin, F.; Bagheri, S.; Arof, A. K.; Abd Hamid, S. B. J Solid State Electrochem. 2012, 16, 3245.
[18] Chen, J. Y.; Zhao, C. X.; Zhi, M. M.; Wang, K. W.; Deng, L. L.; Xu, G. Electrochim. Acta 2012, 66, 133.
[19] Galindo, R.; Mazario, E.; Gutiérrez, S.; Morales, M. P.; Herrasti, P. J. Alloys Compd. 2012, 536S, S241.
[20] Danaee, I.; Jafarian, M.; Forouzandeh, F.; Gobal, F. Int. J. Chem. Kinet. 2012, 44, 712.
[21] El-Refaei, S. M.; Saleh, M. M.; Awad, M. I. J. Power Sources 2013, 223, 125.
[22] Erlebacher, J.; Aziz, M. J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Nature 2001, 410, 450.
[23] Wu, Z. J.; Ge, S. H.; Zhang, M. H.; Li, W.; Mu, S. C.; Tao, K. Y. J. Phys. Chem. C 2007, 111, 8587.
[24] Han, Q.; Liu, K. R.; Chen, J. S.; Li, X.; Wei, X. J. Int. J. Hydrogen Energy 2004, 29, 243.
[25] Zheng, Y. X.; Yao, S. B.; Zhou, S. M. Electrochemistry 2007, 13, 307. (郑一雄, 姚士冰, 周绍民, 电化学, 2007, 13, 307.)
[26] Zheng, Y. X.; Yao, S. B.; Zhou, S. M. Acta Phys. -Chim. Sin. 2008, 24, 1643. (郑一雄, 姚士冰, 周绍民, 物理化学学报, 2008, 24, 1643.)
[27] Jia, F. L.; Yu, C. F.; Deng, K. J.; Zhang, L. Z. J. Phys. Chem. C 2007, 111, 8424.
[28] Shu, Y. D.; Chen, B. C. Metallurge Electrochemistry of Studing Methods, Certral South University of Technology Press, Changsha, 1990, pp. 177~178. (舒佘德, 陈白珍, 冶金电化学研究方法, 中南工业大学出版社, 长沙, 1990, pp. 177~178.)
[29] Zhou, Z.; Yan, J.; Lin, J.; Zhang, Y. S. Chin. J. Power Sources 1999, 23, 319. (周震, 阎杰, 林进, 张允什, 电源技术, 1999, 23, 319.)
[30] Danaee, I.; Jafarian, M.; Forouzandeh, F.; Gobal, F. Int. J. Hydrogen Energy 2009, 34, 859.
[31] Tehrani, R. M. A.; Ab Ghani, S. Fuel Cells 2009, 9, 579.
[32] Laviron, E.; Roullier, L. J. Electroanal. Chem. 1980, 115, 65.
[33] Ojani, R.; Raoof, J. B.; Salmany-Afagh, P. J. Electroanal. Chem. 2004, 571, 1
[34] Shu, Y. D.; Chen, B. C. Metallurge Electrochemistry of Studing Methods, Certral South University of Technology Press, Changsha, 1990, p. 88. (舒佘德, 陈白珍, 冶金电化学研究方法, 中南工业大学出版社, 长沙, 1990, p. 88.)
[35] Bard, A. J.; Faulkner, L. R. Electrochemical Methods, Fundamentals and Applications, Chemical Industry Press, Beijing, 2005, pp. 329~352, translated by Shao, Y. H.; Zhu, G. Y.; Dong, X. D.; Zhang, B. L. (Bard, A. J.; Faulkner, L. R. 电化学方法原理和应用, 邵元华, 朱果逸, 董献堆, 张柏林译, 化学工业出版社, 北京, 2005, pp. 329~352.)
[36] Bard, A. J.; Faulkner, L. R. Electrochemical Methods, Fundamentals and Applications, translated by Shao, Y. H.; Zhu, G. Y.; Dong, X. D.; Zhang, B. L. Chemical Industry Press, Beijing, 2005, p. 566. (Bard, A.J.; Faulkner, L. R. 电化学方法原理和应用, 邵元华, 朱果逸, 董献堆, 张柏林译, 化学工业出版社, 北京, 2005, p. 566.)
[37] Döner, A.; Telli, E.; Kardas, G. J. Power Sources 2012, 205, 71.
/
| 〈 |
|
〉 |