

氧化锌多孔微米花的制备及其气敏性能研究
收稿日期: 2013-12-04
网络出版日期: 2014-02-17
基金资助
项目受国家自然科学基金(Nos.21173115,20873057)资助.
Synthesis and Gas-Sensing Properties of ZnO Porous Microflowers
Received date: 2013-12-04
Online published: 2014-02-17
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21173115, 20873057).
具有大比表面积和丰富表面缺陷的三维多孔纳米材料在气体传感中极具潜力. 通过液相共沉淀法制备了三维微米花状锌基前驱物,于400 ℃热处理即转化为ZnO多孔微米花,它继承了锌基前驱物的外观形貌,但片状“花瓣”的表面形成大量无序孔洞,使得ZnO多孔微米花具有大比表面积(31.3 m2·g-1)和丰富的表面缺陷. 鉴于ZnO多孔微米花的独特微观形貌结构,用其构建的气体传感器对乙醇气体表现出最佳工作温度低、灵敏度高、响应(恢复)快速等优点,与文献报道的ZnO基乙醇气体传感器相比处于领先水平. 结合其制备过程简单、成本低等优点,ZnO多孔微米花在气体传感领域有潜在的应用价值.
熊静芳 , 肖佩 , 吴强 , 王喜章 , 胡征 . 氧化锌多孔微米花的制备及其气敏性能研究[J]. 化学学报, 2014 , 72(4) : 433 -439 . DOI: 10.6023/A13121212
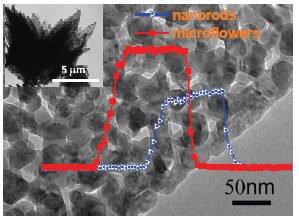
Three-dimensional (3D) porous nanomaterials with large surface area and abundant surface defects have promising potential in gas sensing due to the following merits: (i) large contact area of gaseous species with the materials, and more adsorption sites on the highly defective surface, (ii) good accessibility to gaseous species of the 3D open structure, (iii) fast electron transport among the 3D network. Rational design and controlled synthesis of the 3D porous nanomaterials with specific morphology and microstructure are essential for improving the performance of gas sensing. In this work, 3D ZnO porous microflowers were prepared by directly calcining the zinc-based flowerlike precursor at 400 ℃ in air. The precursor was preformed through a simple coprecipitation method, i.e., by refluxing the aqueous solution of zinc nitrate and co-precipitators of hexamethylenetetramine and oxalic acid at 90 ℃ for 4 h. The unique ZnO porous microflowers were composed of porous nanosheets of 10~50 nanometers in thickness and 1~2 micrometers in width, which inherited from the zinc-based precursor except for the randomly distributed pores on the nanosheet "petals". The 3D porous microstructures endowed ZnO with large specific surface area of 31.3 m2·g-1 and abundant surface defects. ZnO porous microflowers were used as active materials to fabricate gas sensors, which exhibited low working temperature, high sensitivity and fast response (recovery) characteristic against ethanol vapor, at the advanced level in comparison with the reported ZnO-based gas sensors for ethanol. The superior performance of the gas sensors could be attributed to the unique microstructures of the ZnO porous nanomaterials. In addition, the sensitivity of the gas sensors showed an exponential relationship with the concentration of ethanol vapor, indicating their capability of quantitative detection within the ethanol volume ratio of 1×10-6~500×10-6. Together with the superb sensing performance, simple preparation processing and low cost, ZnO porous microflowers have promising application prospect in gas sensing area.

[1] Huang, M. H.; Mao, S.; Feick, H.; Yan, H. Q.; Wu, Y. Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. D. Science 2001, 292, 1897.
[2] Wang, Z. L.; Song, J. H. Science 2006, 312, 242.
[3] Wang, Z. L. Adv. Mater. 2012, 24, 4632.
[4] Law, M.; Greene, L. E.; Johnson, J. C.; Saykally, R. J.; Yang, P. D. Nat. Mater. 2005, 4, 455.
[5] Gao, T.; Wang, T. H. Appl. Phys. A 2005, 80, 1451.
[6] Feng, X. J.; Feng, L.; Jin, M. H.; Zhai, J.; Jiang, L.; Zhu, D. B. J. Am. Chem. Soc. 2004, 126, 62.
[7] Vayssieres, L. Adv. Mater. 2003, 15, 464.
[8] Arnold, M. S.; Avouris, P.; Pan, Z. W.; Wang, Z. L. J. Phys. Chem. B 2003, 107, 659.
[9] Kuo, C. L.; Kuo, T. J.; Huang, M. H. J. Phys. Chem. B 2005, 109, 20115.
[10] Li, J.; Fan, H.; Jia, X. J. Phys. Chem. C 2010, 114, 14684.
[11] Greene, L. E.; Law, M.; Goldberger, J.; Kim, F.; Johnson, J. C.; Zhang, Y. F.; Saykally, R. J.; Yang, P. D. Angew. Chem., Int. Ed. 2003, 42, 3031.
[12] Pan, Z. W.; Dai, Z. R.; Wang, Z. L. Science 2001, 291, 1947.
[13] Zheng, J. H.; Zhang, X. K.; Lu, H. F. Acta Chim. Sinica 2011, 69, 2434. (郑建华, 张晓凯, 卢慧粉, 化学学报, 2011, 69, 2434.)
[14] Bai, H. Y.; Bao, J. C.; Dai, Z. H.; Liu, K. Acta Chim. Sinica 2008, 66, 1786. (白红艳, 包建春, 戴志晖, 刘可, 化学学报, 2008, 66, 1786.)
[15] Zhang, N.; Yu, K.; Li, Q.; Zhu, Z. Q.; Wan, Q. J. Appl. Phys. 2008, 103, 104305.
[16] Son, J. Y.; Lim, S. J.; Cho, J. H.; Seong, W. K.; Kim, H. Appl. Phys. Lett. 2008, 93, 053109.
[17] Law J. B. K.; Thong, J. T. L. Nanotechnology 2008, 19, 205502.
[18] Jing, Z. H.; Zhan, J. H. Adv. Mater. 2008, 20, 4547.
[19] Zuruzi, A. S.; MacDonald, N. C.; Moskovits, M.; Kolmakov, A. Angew. Chem., Int. Ed. 2007, 46, 4298.
[20] Chen, M.; Wang, Z.; Han, D.; Gu, F.; Guo, G. J. Phys. Chem. C 2011, 115, 12763.
[21] Zhang, H.; Wu, J. B.; Zhai, C. X.; Du, N.; Ma, X. Y.; Yang, D. R. Nanotechnology 2007, 18, 455604.
[22] Lee, J. H. Sens. Actuators, B 2009, 140, 319.
[23] Fu, M.; Zhou, J.; Xiao, Q. F.; Li, B.; Zong, R. L.; Chen, W.; Zhang, J. Adv. Mater. 2006, 18, 1001.
[24] Ding, G. Q.; Shen, W. Z.; Zheng, M. J.; Fan, D. H. Appl. Phys. Lett. 2006, 88, 103106.
[25] Zhang, W. X.; Yanagisawa, K. Chem. Mater. 2007, 19, 2329.
[26] Song, R. Q.; Xu, A. W.; Deng, B.; Chen, G. Y. Adv. Funct. Mater. 2007, 17, 296.
[27] Gui, Z.; Liu, J.; Wang, Z. Z.; Song, L.; Hu, Y.; Fan, W. C.; Chen, D. Y. J. Phys. Chem. B 2005, 109, 1113.
[28] Xiong, J. F.; Shen, H.; Mao, J. X.; Qin, X. T.; Xiao, P.; Wang, X. Z.; Wu, Q.; Hu, Z. J. Mater. Chem. 2012, 22, 11927.
[29] Korotcenkov, G.; Han, S. D.; Cho, B. K.; Brinzari, V. Critical Rev. Solid State Mater. Sci. 2009, 34, 1.
[30] Waitz, T.; Becker, B.; Wagner, T.; Sauerwald, T.; Kohl, C. D.; Tiemann, M. Sens. Actuators, B 2010, 150, 788.
[31] Wu, Q.; Chen, J. X.; Zhang, F.; Xiao, P.; Lu, Y. N.; Wang, X. Z.; Hu, Z. CrystEngComm 2012, 14, 3397.
[32] Liu, J. F.; Wang, X.; Peng, Q.; Li, Y. D. Adv. Mater. 2005, 17, 764.
[33] Wang, J. X.; Sun, X. W.; Yang, Y.; Huang, H.; Lee, Y. C.; Tan, O. K.; Vayssieres, L. Nanotechnology 2006, 17, 4995.
[34] Wang, X. Z.; Liu, W.; Liu, J. R.; Wang, F. L.; Kong, J.; Qiu, S.; He, C. Z.; Luan, L. Q. ACS Appl. Mater. Interfaces 2012, 4, 817.
[35] Wang, L. W.; Kang, Y. F.; Liu, X. H.; Zhang, S. M.; Huang, W. P.; Wang, S. R. Sens. Actuators, B 2012, 20, 237.
[36] Chen, J.; Li, J.; Li, J. H.; Xiao, G. Q.; Yang, X. F. J. Alloys Compd. 2011, 509, 740.
[37] Ahn, H.; Park, J. H.; Kim, S. B.; Jee, S. H.; Yoon, Y. S.; Kim, D. J. Electrochem. Solid-State Lett. 2010, 13, J125.
[38] Zhang, Z. Y.; Li, X. H.; Wang, C. H.; Wei, L. M.; Liu, Y. C.; Shao, C. L. J. Phys. Chem. C 2009, 113, 19397.
[39] Zhang, J.; Wang, S. R.; Xu, M. J.; Wang, Y.; Zhu, B. L.; Zhang, S. M.; Huang, W. P.; Wu, S. H. Cryst. Growth Des. 2009, 9, 3532.
[40] Wan, Q.; Li, Q. H.; Chen, Y. J.; Wang, Y. H.; He, X. L.; Li, J. P.; Lin, C. L. Appl. Phys. Lett. 2004, 84, 3654.
/
| 〈 |
|
〉 |