

UV/乙酰丙酮法降解脱色蒽醌类染料茜素红
收稿日期: 2014-01-09
网络出版日期: 2014-02-27
基金资助
项目受国家自然科学基金(No.51378254)、江苏省六大人才高峰(No.JNHB-012)和教育部新世纪优秀人才支持计划(No.NCET-10-0489)资助.
Decoloration of Alizarin Red (an Anthraquinone Dye) with the UV/Acetylacetone Process
Received date: 2014-01-09
Online published: 2014-02-27
Supported by
Project supported by the National Natural Science Foundation of China (No. 51378254), the High Level Talents in Six Industries of Jiangsu Province (No. JNHB-012), and the Program for New Century Excellent Talents in University (No. NCET-10-0489).
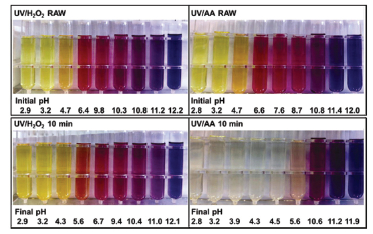
染料废水由于色度高,成份复杂,是一种较难处理的工业废水. 本研究以茜素红为蒽醌类染料的代表,研究了UV/乙酰丙酮(简称UV/AA)法中茜素红浓度、乙酰丙酮用量、溶液初始pH对降解脱色效率的影响. 实验结果表明,UV/AA法在中、酸性条件下对茜素红具有显著的降解脱色效果,其脱色过程符合准一级动力学,降解速率常数远高于UV/H2O2法. 基于溶液pH对UV/AA法脱色效果以及乙酰丙酮紫外吸收光谱的影响,推测在UV/AA法降解脱色染料的过程中起主导作用的是乙酰丙酮的烯醇式异构体. 尽管UV/AA法对总有机碳和化学耗氧量的去除率不高,但是显著提高了溶液的可生化性. 因此,UV/AA法有望作为预处理工艺与传统的生物处理法相结合,以较低的成本实现染料废水的达标处理. 这一工作为小分子双酮在染料废水处理中的应用研究提供了新的思路.
宋孝杰 , 吴兵党 , 张淑娟 . UV/乙酰丙酮法降解脱色蒽醌类染料茜素红[J]. 化学学报, 2014 , 72(4) : 461 -466 . DOI: 10.6023/A14010027
The treatment of dying wastewater, especially the decoloration of it, remains a difficult task in industrial wastewater treatment. Our previous work reports that a novel method, the UV/acetylacetone (AA) process, is efficient in the decoloration of dyes, especially azo dyes. In this work, the decoloration of an anthraquinone dye with the UV/AA method was systematically investigated with Alizarin Red (AR) as a model compound. The effects of initial solution pH, concentration of AR, and concentration of AA on the decoloration efficiency were studied. The AR concentration used in this work was close to the upper limit of actual dye wastewater. Compared with the well-known UV/H2O2 process, the UV/AA process had much higher decoloration efficiency in the pH range of 3~9 and the decoloration process could be described with the pseudo-first order kinetics. Under identical conditions, the k1 of the UV/AA process was 18 times higher than that of the UV/H2O2 process (0.1312 vs 0.0068 min-1). From pH effect experiments, we found that strongly alkaline condition was adverse to the effectiveness of the decoloration. Based on the pH effects on the forms of AA and the UV-Vis spectra of the solutions, we infer that the enol form of AA played a key role in the degradation of AR. Through TOC (total organic carbon), COD (chemical oxygen demand) and BOD (biochemical oxygen demand) analysis, we found that there was only limited removal of the TOC and COD of the solution after the UV/AA treatment. However, the biodegradability of the solution was significantly improved as the BOD/COD was raised from 0.42 to 0.70. This result suggests that the UV/AA process might be used as a pretreatment step in sequential chemical-biological treatment, which provides a new idea for the use of small molecular diketones in wastewater treatment. Interestingly, in the UV/AA process, the decoloration of azo dyes was found to be insensitive to dissolved oxygen, whereas for the decoloration of AR, dissolved oxygen played a crucial role. These results demonstrate that the mechanisms in the UV/AA process for the decoloration of variant types of dyes might be different. Therefore, a quantitative structure-activity relationship is warranted for the better understanding of the UV/AA process.

Key words: decoloration; photodegradation; anthraquinone dye; Alizarin Red; UV/H2O2
[1] Ren, N. Q.; Zhou, X. J.; Guo, W. Q.; Yang, S. S. Chin. J. Chem. Eng. 2013, 64, 84. (任南琪, 周显娇, 郭婉茜, 杨珊珊, 化工学报, 2013, 64, 84.)
[2] Tanaka, K.; Padermpole, K.; Hisanaga, T. Water Res. 2000, 34, 327.
[3] Yoo, E.; Libra, J.; Adrian, L. J. Environ. Eng. 2001, 127, 844.
[4] Lei, P. X.; Chen, C. C.; Ma, W. H.; Zhao, J. C. Acta Chim. Sinica 2005, 63, 1551. (雷鹏翔, 陈春城, 马万红, 赵进才, 化学学报, 2005, 63, 1551.)
[5] Chen, C. C.; Ma, W. H.; Zhao, J. C. Chem. Soc. Rev. 2010, 39, 4206.
[6] Marin, M. L.; Santos-Juanes, L.; Arques, A.; Amat, A. M.; Miranda, M. A. Chem. Rev. 2012, 112, 1710.
[7] Konstantinou, I. K.; Albanis, T. A. Appl. Catal. B 2004, 49, 1.
[8] Baxendale, J.; Wilson, J. Trans. Faraday Soc. 1957, 53, 344.
[9] Wang, M. S.; Liu, X. T.; Pan, B. C.; Zhang, S. J. Chemosphere 2013, 93, 2877.
[10] Tan, Y. M.; Xu, R. F.; Hu, W. K.; Zhang, P. Chin. J. Process Eng. 2004, 4, 234. (谭益民, 徐瑞芬, 胡伟康, 张鹏, 过程工程学报, 2004, 4, 234.)
[11] Tu, D. H.; Li, P.; Shi, C. H. Techniques and Equipment for Environmental Pollution Control 2004, 5, 30. (涂代惠, 李萍, 史长林, 环境污染治理技术与设备, 2004, 5, 30.)
[12] Zhang, S. J.; Liu, X. T.; Wang, M. S.; Wu, B. D.; Pan, B. C.; Yang, H.; Yu, H. Q. Environ. Sci. Technol. Lett. 2014, 1, 167.
[13] Liu, X. T.; Song, X. J.; Zhang, S. J.; Wang, M. S.; Pan, B. C. Phys. Chem. Chem. Phys. 2014, 16, 7571.
[14] Lei, L. C.; Wang, D. H. Advanced Oxidation Processes for Water Treatment, Chemical Industry Press, Beijing, 2001, pp. 198~200. (雷乐成, 汪大翚, 水处理高级氧化技术, 化学工业出版社, 北京, 2001, pp. 198~200.)
[15] Stylidi, M. Appl. Catal. B 2004, 47, 189.
[16] Mofaddel, N.; Bar, N.; Villemin, D.; Desbène, P. L. Anal. Bioanal. Chem. 2004, 380, 664. Chen, B. Y.; Hsueh, C. C.; Liu, S. Q.; Hung, J. Y.; Qiao, Y.; Yueh, P. L.; Wang, Y. M. Int. J. Hydrogen Energy 2013, 38, 15598.
/
| 〈 |
|
〉 |