

十二烷基硫酸钠与甜菜碱在气液和油水界面的复配协同作用研究
收稿日期: 2014-02-22
网络出版日期: 2014-04-16
基金资助
项目受国家自然科学基金(No. 21173134)和国家科技重大专项(No. 2008ZX05011)资助.
Study of the Synergistic Effect of Sodium Dodecyl Sulfate and Betaine at the Air/Water and Oil/Water Interfaces
Received date: 2014-02-22
Online published: 2014-04-16
Supported by
Project supported by the National Natural Science Foundation of China (No. 21173134) and the National Major Science and Technology Project (No. 2008ZX05011).
李亚娉 , 吕韦钦 , 曹绪龙 , 宋新旺 , 王其伟 , 李英 . 十二烷基硫酸钠与甜菜碱在气液和油水界面的复配协同作用研究[J]. 化学学报, 2014 , 72(5) : 615 -623 . DOI: 10.6023/A14020124
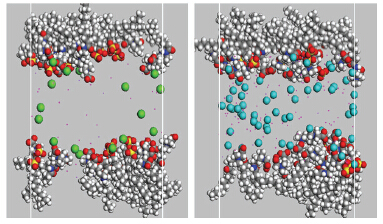
In this paper, the molecular array behavior of anionic surfactant sodium dodecyl sulfate SDS and zwitterionic surfactant Betaine at the air/water and oil/water interfaces were investigated by molecular simulation approaches, which helped to understand the effects of temperature, salts and the proportion of components on the interfacial activity and foam stability of the mixed binary systems, especially how the multivalent inorganic cations influence the interfacial adsorption behavior of the surfactants and the synergistic effect. The oil/water interfacial tension was not only measured experimentally using TEXAS500 spinning drop interface tension meter, but also calculated theoretically using dissipative particle dynamic (DPD) method. Foam decay method was utilized to determine the foam stability. The array behavior of surfactant molecules at the air/water and oil/water interfaces was described by molecular dynamics (MD) simulation method. It was found that the oil/water interfacial activity of mixed binary system was significantly better than unitary system. The synergistic effect between SDS and Betaine was enhanced when there was Ca2+ or Mg2+ existed in the solution. The radial distribution function of head groups of surfactants around inorganic ions showed that there was very strong interaction between the Ca2+ or Mg2+ and the head groups of SDS and Betaine, which not only induced the increase of the maximum interfacial adsorption quantity of the surfactants, but also adjusted the array states of the surfactant molecules in the interface layer. When the proportion of components of SDS/Betaine binary system was 4:6, the oil/water interfacial activity and foam stability of the binary system both were very good with Ca2+ or Mg2+ existing, even use sea water as medium, which could be a good candidate system for foam system used under high salinity condition. The co-adsorption behavior of the surfactant molecules in the binary system at oil/water interface and the foam films was found to be similar. The simulation results agreed well with the experiment results. The knowledge about the microscopic character of the mixtures of surfactants at interfaces could provide useful guidance for the design and application of surfactants under the condition of high salinity, such as low tension foam flooding system used in offshore EOR.
[1] Jing, Z.-S. Overview of Surface Active Agent, China Light Industry Press, Beijing, 1999, pp. 191~242. (荆忠胜, 表面活性剂概论, 中国轻工业出版社, 北京, 1999, pp. 191~242.)
[2] Malysa, K. Adv. Colloid Interface Sci. 1992, 40(2), 37.
[3] Sánchez, C. C.; Patino, J. M. R. Food Hydrocolloids 2005, 19(3), 407.
[4] Farajzadeh, R.; Andrianov, A.; Zitha, P. L. J. Ind. Eng. Chem. Res. 2010, 49(4), 1910.
[5] Yan, Y.-L.; Zhang, N.-S.; Qu, C.-T.; Liu, L.; Gao, Y.-L. Acta Chim. Sinica 2006, 64(1), 54. (燕永利,张宁生,屈撑囤,刘立,高永利, 化学学报, 2006, 64(1), 54.)
[6] Li, Y.; He, X.-J.; Cao, X.-L.; Zhao, G.-Q.; Tian, X.-X.; Cui, X.-H. J. Colloid Interface Sci. 2007, 307, 215.
[7] Acharya, D. P.; Gutiérrez, J. M.; Aramaki, K.; Aratani, K.; Kunieda, H. J. Colloid Interface Sci. 2005, 291, 236.
[8] Jiang, Y.; Lu, X.-Y.; Chen, H.; Mao, S.-Z.; Liu, M.-L.; Luo, P.-Y.; Du, Y.-R. J. Phys. Chem. B 2009, 113, 8357.
[9] Szymczyk, K.; Jańczuk, B. J. Colloid Interface Sci. 2006, 303, 319.
[10] Dar, A. A.; Chatterjee, B.; Rather, G. M.; Das, A. R. J. Colloid Interface Sci. 2006, 298, 395.
[11] Shiloach, A.; Blankschtein, D. Langmuir 1998, 14(25), 7166.
[12] Hines, J. D.; Thomas, R. K.; Garrett, P. R.; Rennie, G. K. J. Phys. Chem. B 1998, 102, 8834.
[13] Prajapati, R. R.; Bhagwat, S. S. J. Chem. Eng. Data 2012, 57, 3644.
[14] Danov, K. D.; Kralchevska, S. D.; Kralchevsky, P. A.; Ananthapadmanabhan, K. P.; Lips, A. Langmuir 2004, 20, 5445.
[15] Zhang, Z.-Q.; Xu, G.-Y.; Ye, F.; Zheng, L.-Q.; Luan, Y.-X. Acta Phys.-Chim. Sinica 2001, 17(12), 1122. (张志庆, 徐桂英, 叶繁, 郑立强, 栾玉霞, 物理化学学报, 2001, 17(12), 1122.)
[16] Srinivasan, V.; Blankschtein, D. Langmuir 2003, 19, 9946.
[17] Fang, Y.; Xia, Y.-M. China Surfactant Detergent & Cosmetics 1998, (6), 22. (方云, 夏咏梅, 日用化学工业, 1998, (6), 22.)
[18] Xia, J.-D.; Fang, Y.; Zhu, S.-P. The Fourth International Surfactants Congress and Exhibition, Barcelona, 1996
(c), 72.
[19] Li, Y.; He, X.-J.; Cao, X.-L.; Shao,Y.-H.; Li, Z.-Q.; Dong, F.-L. Mol. Simul. 2005, 31(15), 1027.
[20] Hu, X.-Y.; Li, Y.; He, X.-J.; Li, C.-X.; Li, Z.-Q.; Cao, X.-L.; Xin, X.; Somasundaran, P. J. Phys. Chem. B 2012, 116, 160.
[21] Koehler, S. A.; Hilgenfeldt, S.; Stone, H. A. Langmuir 2000, 16, 6327.
[22] Hu, X.-Y.; Song, X.-W.; Li, Q.-W.; He, X.-J.; Wang, Q.-W.; Li, Y. Acta Chim. Sinica 2009, 67(14), 1691. (胡晓莹, 宋新旺, 李全伟,何秀娟, 王其伟, 李英, 化学学报, 2009, 67(14), 1691.)
[23] Bhattacharyya, A.; Monroy, F.; Langevin, D.; Argillier, J. F. Langmuir 2000, 16, 8727.
[24] Li, C.-X.; Li, Y.; Yuan, R.; Lv, W.-Q. Langmuir 2013, 29(18), 5418.
[25] Hoogerbrugge, P. J.; Koelman, J. M. V. A. Europhys. Lett. 1992, 19, 155.
[26] Rekvig, L.; Kranenburg, M.; Vreede, J.; Hafskjold, B.; Smit, B Langmuir 2003, 19(20), 8195.
[27] Zhu, P.-F.; Li, Y.; Li, Q.-W.; Song, X.-W.; Cao, X.-L.; Li, Z.-Q. Acta Chim. Sinica 2011, 69(20), 2420. (朱鹏飞, 李英, 李全伟, 宋新旺, 曹绪龙, 李振泉, 化学学报, 2011, 69(20), 2420.)
[28] Li, Z.-Q.; He, X.-J.; Li, Y.; Ma, B.-M.; Cao, X.-L.; Song, X.-W.; Cui, X.-H. Acta Chim. Sinica 2007, 65(24), 2803. (李振泉, 何秀娟, 李英, 马保民, 曹绪龙, 宋新旺, 崔晓红, 化学学报, 2007, 65(24), 2803.)
[29] Dong, F.-L.; Li, Y.; Zhang, P. Chem. Phys. Lett. 2004, 399, 215.
[30] Berendsen, H. J. C.; Grigera, J. R.; Straatsma, T. P. J. Phys. Chem. 1987, 91, 6269.
[31] Gamba, Z.; Hautman, J.; Shelley, J. C.; Klein, M. L. Langmuir 1992, 8, 3155.
[32] Hu, X.-Y.; Li, Y.; Zhang, H.; He, X.-J.; Xue, Y.-Z.; Wang, P.; Yang, J.-L. Acta Chim. Sinica 2010, 68(2), 131. (胡晓莹, 李英, 张辉, 何秀娟, 薛玉志, 王萍, 杨景利, 化学学报, 2010, 68(2), 131.)
[33] Verlet, L. Phys. Rev. 1967, 159, 98.
[34] Nose, S. J. Chem. Phys. 1984, 81, 511.
[35] Hoover, W. G. Phys. Rev. A 1985, 31, 1695.
/
| 〈 |
|
〉 |