

Fe3+诱导全氟羧酸的光化学降解:pH值及链长的影响
收稿日期: 2014-02-13
网络出版日期: 2014-05-27
基金资助
项目受贵州大学引进人才项目(No.贵大人基合字(2010)009号)、贵州省自然科学基金(No.黔科合J字[2011]2066号)、国家环境保护大气复合污染来源与控制重点实验室开放基金(No.SCAPC201302)和国家自然科学基金(No.21267006)资助.
Photochemical Degradation of Perfluorocarboxylic Acids Induced by Ferric Ion:Effects of pH and Carbon Chain
Received date: 2014-02-13
Online published: 2014-05-27
Supported by
Project supported by Guizhou University Talent Introduction Project (No.2010009), Natural Science Foundation of Guizhou Province (No.20112066), State Environmental Protection Key Laboratory of Sources and Control of Air Pollution Complex (No.SCAPC201302) and National Natural Science Foundation of China (No.21267006).
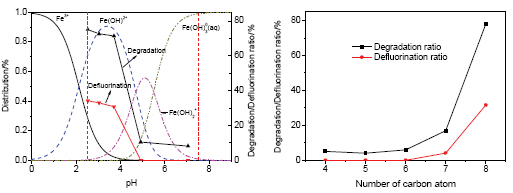
以全氟辛酸(PFOA)为代表的全氟化合物是环境水体中新出现的一类持久性有机污染物,Fe3+的存在促进了其在254 nm紫外光下的有效降解.在此基础上,主要考察了溶液初始pH值对Fe3+诱导PFOA光化学降解的影响,并以全氟丁酸(PFBA)、全氟戊酸(PFPeA)、全氟己酸(PFHxA)和全氟庚酸(PFHpA)为对象,研究了Fe3+诱导短链全氟羧酸(PFCAs)的降解,通过对降解中间产物的分析,进而推断了其降解机理.结果表明,强酸性条件有利于PFOA的降解,弱酸性或中性反应条件下,PFOA的降解和脱氟均受到明显地抑制,进一步证实PFOA的降解主要是溶解性铁作用的结果,此时Fe(OH)2+则是铁(III)-羟基配合物的主要分配形态.Fe3+诱导PFCAs的降解表明: 当碳原子数大于5,长链的PFCAs更易于降解,但对于碳原子数小于6的PFCAs,其降解没有明显的规律.降解中间产物主要是链更短的PFCAs,由此推断,PFCAs的降解遵循逐级降解的规律.
王媛 , 石晓燕 . Fe3+诱导全氟羧酸的光化学降解:pH值及链长的影响[J]. 化学学报, 2014 , 72(6) : 682 -688 . DOI: 10.6023/A14020101
Perfluorooctanoic acid (PFOA), as a typical perfluorinated compounds, is one kind of emerging persistent pollutants appearing in natural water.The existence of ferric ion in aquatic system improves the degradation of PFOA under the irradiation of 254 nm UV light.pH is an important factor for not only the distribution of photo activity species of ferric ion but also the degradation of PFOA.When the initial pH of reaction solution was 2.53, 3.04 and 3.71, respectively, PFOA was decomposed effectively and no obvious difference was observed whether for the degradation ratio of PFOA or for its corresponding defluorination ratio.On the contrary, when pH was 4.92 and 7.05, respectively, the degradation and defluorination of PFOA was inhibited markedly, which further confirmed that dissoluble iron played a key role in the decomposition of PFOA.And Fe(OH)2+ was the dominant species of the hydroxy complexes of Fe3+.At the same time, the degradation of short chain PFCAs bearing C4~C7 caused by ferric ions under the irradiation of 254 nm UV light was also investigated.As for PFCAs with C6~C8, the degradation ratio at 240 min was as follows: PFOA > PFHpA > PFHxA, which showed that the longer the carbon chain was, the easier the degradation of PFCAs was.While, for PFCAs with the number of carbon element less than 6, their degradation ratio at 240 min was as follows: PFHxA > PFBA > PFPeA.The relationship between the degradation of PFCAs and the length of carbon chain of PFCAs was not clear.More short chain PFCAs were identified by LC/MS as the primary intermediates of these short chain PFCAs.Based on these, it was proposed that the degradation of PFCAs bearing C4~C8 was mainly caused by the formation of complexes between ferric ion and PFCAs, and then hydroxyl radical formed from photolysis of Fe(OH)2+ and the redox cycle of Fe2+/Fe3+ further improved their degradation.As a result, PFCAs were decomposed step by step.

[1] Rayne, S.; Forest, K.J.Environ.Sci.Heal.C 2009, 44, 1145.
[2] Holzer, J.; Midasch, O.; Rauchfuss, K.; Kraft, M.; Reupert, R.; Angerer, J.; Kleeschulte, P.P.; Marschall, N.; Wilhelm, M.Environ.Health Persp.2008, 116, 651.
[3] Lynda, A.N.; John, M.N.; Frances, S.S.; Nancy, V.R.; Edward, A.E.Reprod.Toxicol.2009, 27, 231.
[4] Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L.Environ.Sci.Technol.2011, 45, 7954.
[5] Schroder, H.F.; Meesters, R.J.W.J.Chromatogr.A 2005, 1082, 110.
[6] Dillert, R.; Bahnemann, D.; Hidaka, H.Chemosphere 2007, 67, 785.
[7] Song, C.; Chen, P.; Wang, C.; Zhu, L.Chemosphere 2012, 86, 853.
[8] Park, H.; Vecitis, C.D.; Cheng, J.; Choi, W.; Mader, B.T.; Hoffmann, M.R.J.Phys.Chem.A 2009, 113, 690.
[9] Yamamoto, T.; Noma, Y.; Sakai, S.; Shibata, Y.Environ.Sci.Technol.2007, 41, 5660.
[10] Wang, Y.; Zhang, P.Y.; Pan, G.; Chen, H.J.Hazard.Mater.2008, 160, 181.
[11] Wang, Y.; Zhang, P.Y.J.Hazard.Mater.2011, 192, 1869.
[12] Wang, Y.; Zhang, P.Y.Acta Chim.Sinica 2010, 68, 345.(王媛, 张彭义, 化学学报, 2010, 68, 345).
[13] Dillert, R.; Bahnemann, D.; Hidaka, H.Chemosphere 2007, 67, 785.
[14] Panchangam, S.C.; Lin, A.Y.C.; Shaik, K.L.; Lin, C.F.Chemosphere 2009, 77, 242.
[15] Lee, Y.C.; Lo, S.I.; Kuo, J.; Hsieh, C.H.Front.Environ.Sci.Eng.2012, 6, 17.
[16] Deng, N.S.; Wu, F.Environmental Photochemistry, Chemical Industry Press, Beijing, 2003, pp.83~101.(邓南圣, 吴锋, 环境光化学, 化学工业出版社, 北京, 2003, pp.83~101).
[17] Martin, J.W.; Mabury, S.A.; Solomon, K.R.; Muir, D.C.G.Environ.Toxicol.Chem.2003, 22, 189.
[18] Rebecca, R.Environ.Sci.Technol.2006, 40, 12.
[19] Sun, H.W.; Li, F.S.; Zhang, T.; Zhang, X.Z.; He, N.; Song, Q.; Zhao, L.J.; Sun, L.N.; Sun, T.H.Water Res.2011, 45, 4483.
[20] Quinete, N.; Wu, Q.; Zhang, T.; Yun, S.H.; Moreira, I.; Kannan, K.Chemosphere 2009, 77, 863.
[21] Naile, J.E.; Khim, J.S.; Wang, T.Y.; Chen, C.L.; Luo, W.; Kwon, B.; Park, J.; Koh, C.H.; Jones, P.D.; Lu, Y.L.; Giesy, J.P.Environ.Pollut.2010, 158, 1237.
[22] Mak, Y.L.; Taniyasu, S.; Yeung, L.W.Y.; Lu, G.H.; Jin, L.; Yang, Y.L.; Lam, P.K.S.; Kannan, K.; Yamashita, N.Environ.Sci.Technol.2009, 43, 4824.
[23] Stefannson, A.Environ.Sci.Technol.2007, 41, 6117.
[24] Dean, J.A.Lange’s Handbook of Chemistry, 15th ed., McGraw-Hill Inc., New York, 1999, p.1195.
[25] Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R.Front.Environ.Sci.Eng.2009, 3, 129.
[26] Niu, J.F.; Lin, H.; Gong, C.; Sun, X.M.Environ.Sci.Technol.2013, 47, 14341.
[27] Niu, J.F.; Lin, H.; Gong, C.; Sun, X.M.Environ.Sci.Technol.2012, 46, 10191.
[28] Zhuo, Q.F.; Deng, S.B.; Yang, B.; Huang, J.; Wang, B.; Zhang, T.T.; Yu, G.Electrochim.Acta 2012, 77, 17.
[29] Taniyasu, S.; Yamashita, N.; Yamazaki, E.; Petrick, G.; Kannan, K.Chemosphere 2013, 90, 1686.
[30] Hori, H.; Yamamoto, A.; Koike, K.; Kutsuna, S.; Osaka, I.; Arakawa, R.Chemosphere 2007, 68, 572.
/
| 〈 |
|
〉 |