

Pd催化木质素醚类二聚体分子内氢转移断裂C—O键研究
收稿日期: 2014-05-22
网络出版日期: 2014-09-10
基金资助
项目受973计划(Nos. 2012CB215306,2013CB228103)、国家自然科学基金(Nos. 21325208,21361140372,21172209)、教育部中央高校基本科研业务费专项(No. WK2060190025)、高等学校博士点基金(No. 20123402130008)、中国科学院基金(No. KJCX2-EW-J02)和霍英东教育基金资助.
Supported Pd Catalysts for the C—O Cleavage of the Lignin Derived Model Dimers through Intramolecular Hydrogenolysis Reaction
Received date: 2014-05-22
Online published: 2014-09-10
Supported by
Project supported by the National Basic Research Program of China (Nos. 2012CB215306, 2013CB228103), the National Natural Science Foundation of China (No. 21325208, 21361140372, 21172209), the Fundamental Research Funds for the Central Universities (No. WK2060190025), the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20123402130008), Chinese Academy of Sciences (No. KJCX2-EW-J02) and Fok Ying Tung Education Foundation.
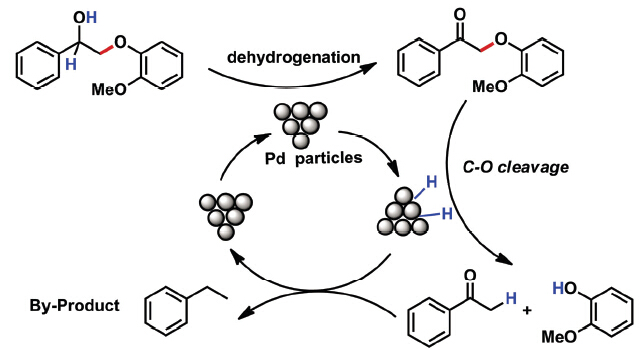
结合氢转移方法, 研究了木质素模型物2-(2'-甲氧基苯氧基)-1-苯乙醇(1a)分子在无外加氢源的条件下利用金属钯催化剂催化发生C—O键断裂反应. 合成并表征了一系列Pd负载型催化剂, 通过优化发现反应体系在环己烷溶剂和弱碱添加剂Na2HPO4条件下显示出较好的催化效率. 结合反应特点将催化剂进行改进, 使用MgO作为载体的催化剂Pd/MgO高效完成了木质素模型物的分子自供氢降解. 反应过程可能分为两步进行: 首先, 模型物在钯表面先进行脱氢过程, 含羟基的木质素模型物二聚体1a脱去氢后生成酮式中间体2-(2'-甲氧基苯氧基)-1-苯乙酮(1b), 被脱去的氢原子吸附于钯表面. 随后, 脱氢中间体1b在Pd催化下与其表面吸附的H作用, 发生催化C—O键断键过程.
严龙 , 庞欢 , 黄耀兵 , 傅尧 . Pd催化木质素醚类二聚体分子内氢转移断裂C—O键研究[J]. 化学学报, 2014 , 72(9) : 1005 -1011 . DOI: 10.6023/A14050397
The selective depolymerization of the C—O ether bonds in lignin has attracted considerable attentions which would facilitate the valorization of lignin. The model compounds with β-O-4 type ether bonds were the most frequently studied molecules. In this study, we used 2-(2-methoxyphenyl)oxy-1-phenethanol (1a) as model compound for the C—O cleavage through intramolecular hydrogenolysis reaction. The hydroxymethyl group acted as the hydrogen source by dehydrogenating over Pd catalysts. Then, the cleavage of C—O bond takes place on the supported Pd catalyst with Pd-H active species. All the catalysts were prepared and characterized by XRD, TEM and BET. Among different metal catalysts, Pd catalysts gave best results toward the C—O bond cleavage reaction. After that, the influences of solvent were studied and the best solvent was cyclohexane. Different additives such as acids and bases were also tested in the model reaction. The strong base (i.e. KOH) showed negative effect on the product yield, but a weak base (i.e. Na2HPO4) gave a promotion effect on the reaction. The result indicated that the modification of the Pd catalysts' supports would be a good option for the C—O bond cleavage reaction. Thus, different Pd catalysts with different supporting materials were prepared and tested, and found that MgO, a weak basic support, gave promoted performance on this reaction. Finally, to figure out the reaction pathway, the reaction mixtures with different reaction time were analyzed by GC-MS which showed that the ketone was the main intermediate of the reaction. The result indicated that the main route for the intramolecular hydrogenolysis reaction was (1) the dehydrogenation of –CCHOH to ketone to form Pd-H species and then (2) the C—O bond was cleavage by the Pd-H species to form the depolymerization products.

[1] Holdren, J. P. Science 2007, 315, 737.
[2] (a) Vispute, T. P.; Zhang, H.; Sanna, A.; Xiao, R.; Huber, G. W. Science 2010, 330, 1222.
(b) Crossley, S.; Faria, J.; Shen, M.; Resasco, D. E. Science 2010, 327, 68.
(c) Zhang, Y. H. P. J. Ind. Microbiol. Biotechnol. 2008, 35, 367.
[3] (a) Regalbuto, J. R. Science 2009, 325, 822.
(b) Holm, M. S.; Saravanamurugan, S.; Taarning, E. Science 2010, 328, 602.
(c) Luo, C.; Wang, S.; Liu, H. Angew. Chem. Int. Ed. 2007, 46, 7636.
(d) Mascal, M.; Nikitin, E. B. Angew. Chem. Int. Ed. 2008, 120, 8042.
(e) Ragauskas, A. J.; Williams, C. K.; Davison, B. H.; Britovsek, G.; Cairney, J.; Eckert, C. A.; Frederick, Jr. W. J.; Hallett, J. P.; Leak, D. J.; Liotta, C. L.; Mielenz, J. R.; Murphy, R.; Templer, R.; Tschaplinski, T. Science 2006, 311, 484.
[4] Zakzeski, J.; Bruijnincx, P. C.; Jongerius, A. L.; Weckhuysen, B. M. Chem. Rev. 2010, 110, 3552.
[5] Chakar, F. S.; Ragauskas, A. J. Ind. Crops Prod. 2004, 20, 131.
[6] Balakshin, M. Y.; Capanema, E. A.; Chang, H. M. Characterization of Lignocellulosic Materials, Blackwell Publishing Ltd., Oxford, UK, 2008, p. 148.
[7] (a) Wang, X.; Rinaldi, R. Angew. Chem. Int. Ed. 2013, 52, 11499.
(b) Wang, X.; Rinaldi, R. ChemSusChem 2012, 5, 1455.
(c) Wang, X.; Rinaldi, R. Energy Environ. Sci. 2012, 5, 8244.
[8] (a) Sergeev, A. G.; Hartwig, J. F. Science 2011, 332, 439.
(b) Sergeev, A. G.; Webb, J. D.; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 20226.
[9] (a) He, J.; Zhao, C.; Lercher, J. A. J. Am Chem Soc. 2012, 134, 20768.
(b) Yan, N.; Zhao, C.; Dyson, P. J.; Wang, C.; Liu, L. T.; Kou, Y. ChemSusChem 2008, 1, 626.
(c) Zhao, C.; Lercher, J. A. ChemCatChem 2012, 4, 64.
(d) Zhao, C.; Lercher, J. A. Angew. Chem. Int. Ed. 2012, 124, 6037.
[10] (a) Zhao, C.; Kou, Y.; Lemonidou, A. A.; Li, X.; Lercher, J. A. Angew. Chem. Int. Ed. 2009, 121, 4047.
(b) Yan, N.; Zhao, C.; Dyson, P. J.; Wang, C.; Liu, L. T.; Kou, Y. ChemSusChem 2008, 1, 626.
(c) Yan, N.; Yuan, Y.; Dykeman, R.; Kou, Y.; Dyson, P. J. Angew. Chem. Int. Ed. 2010, 49, 5549.
[11] Zhang, Q. S.; Wang, L. L. J. Mol. Catal (China) 2013, 27, 89. (张勤生, 王来来, 分子催化, 2013, 27, 89.)
[12] Li, G. X.; Dong, P.; Wang, X. R.; Xu, Y. D.; Wang, C. J.; Liu, Y. J. Mol. Catal (China) 2012, 26, 26. (李贵贤, 董鹏, 王小瑞, 徐彦铎, 王成君, 刘扬, 分子催化, 2012, 26, 26.)
[13] Liang, K. Ph.D. Dissertation, University of Lanzhou, Lanzhou, 2008. (梁琨, 博士论文, 兰州大学, 兰州, 2008.)
[14] Liu, L. T.; Zhang, B.; Li, J.; Ma, D.; Kou, Y. Acta Phys.-Chim. Sin. 2012, 28, 2343. (刘凌涛, 张斌, 李晶, 马丁, 寇元, 物理化学学报, 2012, 28, 2343.)
[15] Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Energy Environ. Sci. 2013, 6, 994.
[16] Molinari, V.; Giordano, C.; Antonietti, M.; Esposito, D. J. Am. Chem. Soc. 2014, 136, 1758.
[17] Ren, Y. L.; Yan, M.; Wang, J. J.; Zhang, Z. C.; Yao, K. S. Angew. Chem. Int. Ed. 2013, 52, 12674.
[18] Kleine, T.; Buendia, J.; Bolm, C. Green Chem. 2013, 15, 160.
[19] Desnoyer, A. N.; Fartel, B.; MacLeod, K. C.; Patrick, B. O.; Smith, K. M. Organometallics 2012, 31, 7625.
[20] (a) Nguyen, J. D.; Matsuura, B. S.; Stephenson, C. R. J. J. Am. Chem. Soc. 2014, 136, 1218.
(b) Lian, Y. F.; Yan, L. L.; Wang, Y.; Qi, X. H. Acta Chim. Sinica 2014, 72, 502. (廉优芬, 闫碌碌, 王羽, 漆新华, 化学学报, 2014, 72, 502.)
[21] (a) Strassberger, Z.; Alberts, A. H.; Louwerse, M. J.; Tanase, S.; Rothenberg, G. Green Chem. 2013, 15, 768.
(b) Zhang, J.; Teo, J.; Chen, X.; Asakura, H.; Tanaka, T.; Teramura, K.; Yan, N. ACS Catal. 2014,4, 1574.
(c) Li, C.; Zheng, M.; Wang, A.; Zhang, T. Energy Environ. Sci. 2012, 5, 6383.
(d) Sturgeon, M. R.; O'Brien, M. H.; Ciesielski, P. N.; Katahira, R.; Kruger, J. S.; Chmely, S. C.; Hamlin, J.; Lawrence, K.; Glendon, B. Hunsinger, G. B.; Foust, T. D.; Baldwin, R. M.; Biddy, M. J.; Beckham G. T. Green Chem. 2014, 16, 824.
(e) Zhou, X.; Mitra, J.; Rauchfuss, T. B. ChemSusChem 2014, 7, 1623.
(f) Harms, R. G.; Markovits, I. I.; Drees, M.; Herrmann, W. A.; Cokoja, M.; Kühn, F. E. ChemSusChem 2014, 7, 429.
[22] Nichols, J. M.; Bishop, L. M.; Bergman, R. G.; Ellman, J. A. J. Am. Chem. Soc. 2010, 132, 12554.
[23] Guo, J. L.; Shen, Y. N. Chinese J. Inner Mongolia Normal University 2009, 38, 357. (郭金玲, 沈岳年, 内蒙古师范大学学报, 2009, 38, 357.)
[24] (a) Tanner, D.; Somfai, P. Tetrahedron 1987, 43, 4395.
(b) Jae, J.; Zheng, W.; Lobo, R. F.; Vlachos, D. G. ChemSusChem 2013, 6, 1158.
[25] Johnstone, R. A. W.; Wilby, A. H.; Entwistle, I. D. Chem. Rev. 1985, 85, 12.
[26] Huang, L.; Zhu, Y.; Huo, C.; Zheng, H.; Feng, G.; Zhang, C.; Li, Y. J. Mol. Catal. A: Chem. 2008, 288, 109.
[27] Zhang, T.; Tan, H. J.; Hong, Y. L.; Shen, L. J. Hangzhou Normal University (Nat. Sci. Ed.) 2012, 11, 7. (张添, 谭华杰, 洪益玲, 沈良, 杭州师范大学学报: 自然科学版, 2012, 11, 7.)
/
| 〈 |
|
〉 |