

制备方法对Fe/NCNTs催化剂结构及费托反应性能的影响
收稿日期: 2014-06-13
网络出版日期: 2014-09-10
基金资助
项目受国家自然科学基金(Nos. 51232003,21473089,21173114,21173115,21203092)、江苏省科技支撑项目(No. BE2012159)、苏州市科技计划项目(No. ZXG2013025)和“973”项目(No. 2013CB932902)资助.
Influence of Preparation Methods on Catalytic Performance of Fe/NCNTs Fischer-Tropsch Catalysts
Received date: 2014-06-13
Online published: 2014-09-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 51232003, 21473089, 21173114, 21173115, 21203092), Jiangsu Province Science and Technology Support Project (No. BE2012159), Suzhou Science and Technology Plan Projects (No. ZXG2013025) and “973” programs (No. 2013CB932902).
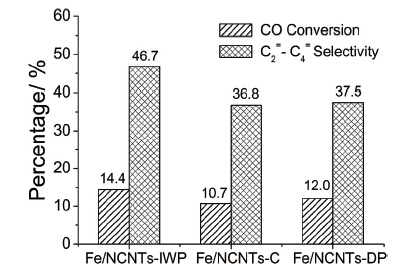
以碳氮纳米管(NCNTs)为载体, 利用氮的锚定作用, 采用三种不同的制备方法(等体积浸渍法、胶体法和沉积沉淀法)方便地构建了负载铁物种的Fe/NCNTs催化剂. 系统考察了制备方法对催化剂的结构及费托反应性能的影响. 结果表明, 制备方法影响铁纳米粒子的粒度和分布、催化剂的还原和碳化行为, 使催化剂表现出不同的催化性能. 等体积浸渍法得到分散性较好、粒径小和分布窄[(8±4) nm]、容易还原和碳化的催化剂, 反应中呈现出最高的低碳烯烃选择性、催化活性和稳定性. 胶体法得到了形貌各异的粒子, 反应中活性相易被氧化使得催化剂活性及稳定性较差. 沉积沉淀法得到了粒径过大、难以还原和碳化的粒子, 反应15 h后催化剂活性及稳定性急剧下降. 该研究为利用氮掺杂碳纳米材料作为载体设计和开发高性能的费托合成催化剂提供了有益参考.
吕金钊, 胡仁之, 卓欧, 许波连, 杨立军, 吴强, 王喜章, 范以宁, 胡征 . 制备方法对Fe/NCNTs催化剂结构及费托反应性能的影响[J]. 化学学报, 2014 , 72(9) : 1017 -1022 . DOI: 10.6023/A14060460
Fischer-Tropsch synthesis (FTS) is a classical topic of great significance because of the approach of post-petroleum times. Recently we found that, taking the advantage of the anchoring effect and intrinsic basicity of nitrogen-doped carbon nanotubes (NCNTs), iron nanoparticles could be conveniently immobilized on the NCNTs without surface pre-modification. The so-constructed Fe/NCNTs catalyst presents the superb catalytic performance in FTS with high selectivity as well as high catalytic activity and stability. In this study, three Fe/NCNTs catalysts were prepared by incipient wetness impregnation method, colloidal method and deposition-precipitation method, denoted as Fe/NCNTs-IWP, Fe/NCNTs-C, and Fe/NCNTs-DP, respectively. The influence of preparation methods on the particle size distribution and morphology, reduction and carbonization of the active species, as well as the FTS catalytic performance were systematically examined. The results indicate that the incipient impregnation method could achieve high dispersion, smaller particles and narrower size distribution[(8±4) nm], leading to the easier reduction and carbonization of the iron nanoparticles compared with those prepared by the other two methods. The FTS catalytic performance of the Fe/NCNTs-IWP catalyst is much better than those of the Fe/NCNTs-C and the Fe/NCNTs-DP catalysts in terms of the high lower olefins selectivity, high catalytic activity and stability. Colloid method got the different morphologies of iron particles with the average size of (13±7) nm. The active iron species was susceptible to oxidation during the reaction, which cause poor catalytic activity and stability. The deposition-precipitation method got the largest particles of (19±11) nm which were difficult to be reduced and carbonized, leading to the sharp decline of the catalytic activity and stability after 15 h reaction. The size-dependence of the catalytic performance for the Fe/NCNTs catalysts in this study are generally in consistent with those in literatures for the iron catalysts supported on carbon nanotubes. These results should be suggestive for exploring the advanced FTS catalysts with the abundant N-doped carbon nanostructures.

[1] Torres Galvis, H. M.; Bitter, J. H.; Khare, C. B.; Ruitenbeek, M.; Dugulan, A. I.; de Jong, K. P. Science 2012, 335, 835.
[2] Torres Galvis, H. M.; de Jong, K. P. ACS Catal. 2013, 3, 2130.
[3] Khodakov, A. Y.; Chu, W.; Fongarland, P. Chem. Rev. 2007, 107, 1692.
[4] Zhang, Q.; Kang, J.; Wang, Y. ChemCatChem 2010, 2, 1030.
[5] Yang, J.; Zhao, B.; Zhao, H.; Lu, A.; Ma, D. Acta Chim. Sinica 2013, 71, 1365. (杨敬贺, 赵博, 赵华博, 陆安慧, 马丁, 化学学报, 2013, 71, 1365).
[6] Xu, L. Y.; Wang, Q. X.; Xu, Y. D.; Huang, J. S. Catal. Lett. 1994, 24,177.
[7] Chen, S.; Li, J.; Zhang, Y.; Zhao, Y.; Liew, K.; Hong, J. Top. Catal. 2014, 57, 437.
[8] Bao, J.; He, J.; Zhang, Y.; Yoneyama, Y.; Tsubaki, N. Angew. Chem., Int. Ed. 2008, 47, 353.
[9] Gao, L.; Xu, Y.; Hou, B.; Wu, D.; Sun, Y.-H. Acta Chim. Sinica 2008, 66, 1851. (高恋, 徐耀, 侯博, 吴东, 孙予罕, 化学学报, 2008, 66, 1851).
[10] Yang, W. S.; Fang, D. Y.; Xiang, H. W.; Li, Y. W. Acta Chim. Sinica 2005, 63, 157. (杨文书,房鼎业,相宏伟,李永旺, 化学学报, 2005, 63, 157).
[11] Chen, W.; Fan, Z. L.; Pan, X. L.; Bao, X. H. J. Am. Chem. Soc. 2008, 130, 9414.
[12] Yang, Z.; Pan, X.; Wang, J.; Bao, X. Catal. Today 2012, 186, 121.
[13] Xu, J.-D.; Zhu, K.-T.; Weng, X.-F.; Weng, W.-Z.; Huang, C.-J.; Wan, H.-L. Catal. Today 2013, 215, 86.
[14] Chen, H.; Yang, Y.; Hu, Z.; Huo, K. F.; Ma, Y. W.; Chen, Y.; Wang, X. S.; Lu, Y. N. J. Phys. Chem. B 2006, 110, 16422.
[15] Jian, G.; Zhao, Y.; Wu, Q.; Yang, L.; Wang, X.; Hu, Z. J. Phys. Chem. C 2013, 117, 7811.
[16] Feng, H.; Ma, J.; Hu, Z. J. Mater. Chem. 2010, 20, 1702.
[17] Yue, B.; Ma, Y. W.; Tao, H. S.; Yu, L. S.; Jian, G. Q.; Wang, X. Z.; Wang, X. S.; Lu, Y. N.; Hu, Z. J. Mater. Chem. 2008, 18, 1747.
[18] Jiang, S. J.; Ma, Y. W.; Jian, G. Q.; Tao, H. S.; Wang, X. Z.; Fan, Y. N.; Lu, Y. N.; Hu, Z.; Chen, Y. Adv. Mater. 2009, 21, 4953.
[19] Jiang, S.; Zhu, L.; Ma, Y.; Wang, X.; Liu, J.; Zhu, J.; Fan, Y.; Zou, Z.; Hu, Z. J. Power Sources 2010, 195, 7578.
[20] Lu, J.; Yang, L.; Xu, B.; Wu, Q.; Zhang, D.; Yuan, S.; Zhai, Y.; Wang, X.; Fan, Y.; Hu, Z. ACS Catal. 2014, 4, 613.
[21] Ndlovu, S. B.; Phala, N. S.; Hearshaw-Timme, M.; Beagly, P.; Moss, J. R.; Claeys, M.; van Steen, E. Catal. Today 2002, 71, 343.
[22] Bahome, M. C.; Jewell, L. L.; Hildebrandt, D.; Glasser, D.; Coville, N. J. Appl. Catal., A 2005, 287, 60.
[23] Guczi, L.; Stefler, G.; Geszti, O.; Koppany, Z.; Konya, Z.; Molnar, E.; Urban, M.; Kiricsi, I. J. Catal. 2006, 244, 24.
[24] Yang, Y.; Jia, L.; Hou, B.; Li, D.; Wang, J.; Sun, Y. ChemCatChem 2014, 6, 319.
[25] Yang, C.; Zhao, H.; Hou, Y.; Ma, D. J. Am. Chem. Soc. 2012, 134, 15814.
[26] Kang, J.; Zhang, S.; Zhang, Q.; Wang, Y. Angew. Chem., Int. Ed. 2009, 48, 2565.
[27] Park, J. Y.; Lee, Y. J.; Khanna, P. K.; Jun, K. W.; Bae, J. W.; Kim, Y. H. J. Mol. Catal. A 2010, 323, 84.
[28] Torres Galvis, H. M.; Bitter, J. H.; Davidian, T.; Ruitenbeek, M.; Dugulan, A. I.; de Jong, K. P. J. Am. Chem. Soc. 2012, 134, 16207.
/
| 〈 |
|
〉 |