

含负氢配体的单核钌催化的降冰片烯开环易位聚合催化研究
收稿日期: 2014-10-09
网络出版日期: 2014-10-10
基金资助
项目受国家自然科学基金(No. 21274058)和国家重点基础研究发展计划(No. 2011CB935801)资助.
Studies on Ring-Opening Metathesis Polymerization of Norborene Catalyzed by Hydride Containing Mononuclear Ruthenium Complex
Received date: 2014-10-09
Online published: 2014-10-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 21274058) and the National Basic Research Program of China (No. 2011CB935801).
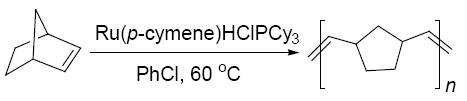
设计的含负氢配体的单核钌配合物Ru(p-cymene)HClPCy3高活性地催化了降冰片烯开环易位聚合反应(ROMP), 并通过设计的Ru(p-cymene)H2PCy3对降冰片烯的催化活性研究证明了单核催化剂需同时含有氯原子配体, 提出了反应机理.
关键词: 烯烃开环易位聚合反应; 降冰片烯; 负氢配体
陈锦森 , 朱聪之 , 陈勋 , 王金虎 , 朱进 . 含负氢配体的单核钌催化的降冰片烯开环易位聚合催化研究[J]. 化学学报, 2014 , 72(11) : 1144 -1146 . DOI: 10.6023/A14100695
Ring-opening metathesis polymerization (ROMP) has emerged as an important tool for the preparation of architecturally unique macromolecules from cyclic olefins. A wide variety of catalytic systems, ranging from simple metal salts to highly sophisticated alkylidene metal complexes, have been used for the achievement of this synthetically useful transformation. Especially, tremendous efforts have been directed toward the ruthenium-based catalytic systems as well as the understanding of their reactivity patterns. And Grubbs catalysts represent the ultimate in terms of activity, but they are not cost effective due to the sophisticated ligand. Therefore, alternative catalyst precursors that are more readily accessible have been and are still being actively developed. Therefore, a hydride ligand containing mononuclear ruthenium complex Ru(p-cymene)HClPCy3 reported here was designed to catalyze ring-opening metathesis polymerization of norbornene with high activity. The hydride ligand is critical based on its high trans effect (for ligand dissociation) and the ability to generate carbene species through plausible migratory insertion and α-elimination steps. This catalytic system bearing hydride ligand could be an alternative for the well-defined Ru-based initiators which rules out the need of installation of complex ancillary ligands like NHC or O-Ligand in the coordination sphere or the need of activation with diazo compounds in the search for novel active catalytic species. Further study on the catalytic polymerization activity of Ru(p-cymene)H2PCy3 compared to the Ru(p-cymene)HClPCy3 system revealed that chloride ligand was also crucial to the hydride containing mononuclear ruthenium system, which contains easily dissociated ligand p-cymene and large phosphine ligand that stabilizes the metal complex. And the possible mechanism for the reaction was proposed.

[1] Sutthasupa, S.; Shiotsuki, M.; Sanda, F. Polym. J. 2010, 42, 905.
[2] Bielawski, C. W.; Grubbs, R. H. Prog. Polym. Sci. 2007, 32, 1.
[3] Black, G.; Maher, D.; Risse, W. In Handbook of Metathesis: Catalyst Development, Ed.: Grubbs, R. H., Wiley-VCH Verlag GmbH, 2008, pp. 2~71.
[4] Buchmeiser, M. R. Chem. Rev. 2000, 100, 1565.
[5] Schwab, P.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1996, 118, 100.
[6] Schwab, P.; France, M. B.; Ziller, J. W.; Grubbs, R. H. Angew. Chem. Int. Ed. Engl. 1995, 34, 2039.
[7] Scholl, M.; Ding, S.; Lee, C. W.; Grubbs, R. H. Org. Lett. 1999, 1, 953.
[8] Harrity, J. P. A.; La, D. S.; Cefalo, D. R.; Visser, M. S.; Hoveyda, A. H. J. Am. Chem. Soc. 1998, 120, 2343.
[9] Garber, S. B.; Kingsbury, J. S.; Gray, B. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2000, 122, 8168.
[10] Romulus, J.; Patel, S.; Weck, M. Macromolecules 2012, 45, 70.
[11] Park, H.; Choi, T.-L. J. Am. Chem. Soc. 2012, 134, 7270.
[12] Lai, W.-Y.; Balfour, M. N.; Levell, J. W.; Bansal, A. K.; Burn, P. L.; Lo, S.-C.; Samuel, I. D. W. Macromolecules 2012, 45, 2963.
[13] Keitz, B. K.; Fedorov, A.; Grubbs, R. H. J. Am. Chem. Soc. 2012, 134, 2040.
[14] Qian, Y.; Chen, B.; Jin, J.; Huang, J. Acta Chim. Sinica 2000, 58, 1050. (钱延龙, 陈斌, 金军挺, 黄吉玲, 化学学报, 2000, 58, 1050.)
[15] Demonceau, A.; Stumpf, A. W.; Saive, E.; Noels, A. F. Macromolecules 1997, 30, 3127.
[16] Delaude, L.; Demonceau, A.; Noels, A. F. Macromolecules 1999, 32, 2091.
[17] Jan, D.; Delaude, L.; Simal, F.; Demonceau, A.; Noels, A. F. J. Organomet. Chem. 2000, 606, 55.
[18] Furstner, A.; Ackermann, L. Chem. Commun. 1999, 95.
[19] Delaude, L.; Demonceau, A.; Noels, A. F. Chem. Commun. 2001, 986.
[20] Louie, J.; Grubbs, R. H. Angew. Chem. Int. Ed. 2001, 40, 247.
[21] Delaude, L.; Szypa, M.; Demonceau, A.; Noels, A. F. Adv. Synth. Catal. 2002, 344, 749.
[22] Chen, J.; Chen, X.; Zhu, C.; Zhu, J. J. Mol. Catal. A-Chem. 2014, 394, 198.
[23] Solari, E.; Gauthier, S.; Scopelliti, R.; Severin, K. Organometallics 2009, 28, 4519.
[24] Demerseman, B.; Mbaye, M. D.; Sémeril, D.; Toupet, L.; Bruneau, C.; Dixneuf, P. H. Eur. J. Inorg. Chem. 2006, 2006, 1174.
/
| 〈 |
|
〉 |