

功能性离子液体萃取水溶液中Cu2+:实验与理论
收稿日期: 2014-11-12
网络出版日期: 2015-01-29
基金资助
项目受国家自然科学基金(Nos. 21103047, 21136004)和中央高校基本科研业务费资助(No. 222201313001).
Extraction of Copper from Aqueous Solution with Functional Ionic Liquids: Experiment and Theoretical Calculation
Received date: 2014-11-12
Online published: 2015-01-29
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21103047, 21136004) and the Fundamental Research Funds for the Central Universities of China (No. 222201313001).
本工作针对含硫脲基咪唑憎水功能离子液体在溶液中Cu2+萃取方面的应用及其机理进行研究. 考察了萃取两相体积比、金属离子浓度、时间、无机盐NaCl、溶液pH及离子液体烷基链长等因素的影响. 结果表明: 室温条件下, 0.1 mL离子液体[CnMPSM][PF6] (n=4、6、8)与5 mL 21.94 mg/L的氯化铜溶液室温条件下超声混合30 min, 溶液中Cu2+的去除率即超过95%; 且此类离子液体对金属离子的萃取效果顺序为: n=4≈n=6>n=8. 以[HMPSM][PF6]为研究对象, 发现溶液中无机盐NaCl的含量以及溶液pH 对金属离子的萃取效果影响不明显. 与传统离子液体[Cnbim][PF6] (n=6、8)相比, 硫脲基的引入使其萃取率由20%左右提高到99%, 且有效避免因阳离子交换而引起水中咪唑阳离子含量增加问题. 通过理论计算发现, 功能离子液体对金属离子的萃取依赖于官能团中的S元素与Cu2+之间较强的静电及路易斯酸碱作用, 与萃取实验中离子液体未和Cu2+发生阳离子交换作用相吻合.
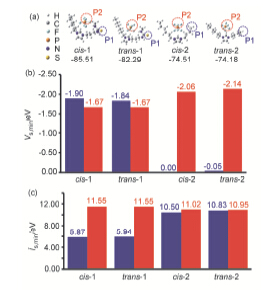
刘梦莹 , 车佳宁 , 吴蔚闳 , 卢运祥 , 彭昌军 , 刘洪来 , 卢浩 , 杨强 , 汪华林 . 功能性离子液体萃取水溶液中Cu2+:实验与理论[J]. 化学学报, 2015 , 73(2) : 116 -125 . DOI: 10.6023/A14110779
This work studied the extraction of copper (II) ions from aqueous solution with thiourea-appended imidazolium hydrophobic ionic liquids and the extraction mechanism by experiment and theory. The influence of parameters affecting the extraction of copper ion, such as the metal ion concentrations, volume ratio between aqueous solution and ionic liquid, contact time, sodium chloride and pH, as well as alkyl chain length was analyzed. In the case of room temperature, the volume ratio between aqueous/IL phases was 50, the extraction efficiencies >95% could be obtained for copper ion with all the ionic liquids [CnMPSM][PF6] (n=4, 6, 8). The results also suggest that n=4 did the same work on the extraction efficiency with n=6 but higher than n=8, while pH and the salt in the solution had little effect on the extraction efficiency for the extraction with [HMPSM][PF6]. The grafted functional group significantly enhanced the extraction efficiency for Cu2+ from 20% to over 99% compared with traditional ILs. To the IL [HMPSM][PF6], the content of imidazolium cation in the aqueous solution before and after extraction with functional IL reduces from 1.24% to 0.85% which means the coordination effect between functional group and the metal ion restrains the release of [HMPSM]+ cation from functional IL to the aqueous solution, while it increases from 0.63% to 0.87% for traditional IL, which is consistent with the results caused by the cation exchange mechanism. In the theory part, the lanl2dz-ECP basis set was employed for transition metal Cu, whereas for the remaining atoms 6-31G (d,p) was applied, cation-anion interaction energies of the ILs and the binding energies between Cu (II) ion and the ILs were calculated, also the surface properties most-negative-surface electrostatic potential (Vs,min) and the lowest surface average local ionization energy (īs,min), were determined by the Multiwfn 2.4 program. All the calculation results show that the sulfur atom from cation is easier to attract the metal ion electrostatically and covalently, thus leads to the high efficiency of extraction.

Key words: thiourea-appended; ionic liquids; extraction; copper; calculation
[1] Kenntner, N.; Krone, O.; Altenkamp, R.; Tataruch, F. Arch. Environ. Contam. Toxicol. 2003, 45, 128.
[2] Swarup, D.; Patre, R. C. Indian J. Anim. Sci. 2005, 75, 231.
[3] Shao, Y.; Ma, Y. Acta Chim. Sinica 2012, 70, 1957. (邵悦, 马勇, 化学学报, 2012, 70, 1957.)
[4] Lemos, A.; Santos, S. Spectrochim. Acta B 2007, 62, 4.
[5] Thakkar, R.; Chudasama, U. J. Hazard. Mater. 2009, 172, 129.
[6] Azeem, S. A.; Arafa, W. A.; El-Shahat, M. F. J. Hazard. Mater. 2010, 182, 286.
[7] Kim, Y. H.; Kim, G. Y.; Lim, H. B. Bull. Korean Chem. Soc. 2010, 31, 908.
[8] Saha, B.; Chakraborty, S.; Das, G. J. Phys. Chem. C 2010, 114, 9817.
[9] Stojanovic, A.; Keppler, B. K. Sep. Sci. Technol. 2012, 47, 189.
[10] Zargoosh, K.; Abedini, H.; Abdolmaleki, A.; Molavian, M. R. Ind. Eng. Chem. Res. 2013, 52, 14944.
[11] Kifle, D.; Wibetoe, G.; Froseth, M.; Bigelius, J. Solvent Extr. Ion Exch. 2013, 3, 1668.
[12] Fan, J.; Fan, Y. C.; Wang, J. J.; Cui, F. L. Acta Chim. Sinica 2006, 64, 1495. (樊静, 范云场, 王键吉, 崔凤灵, 化学学报, 2006, 64, 1495.)
[13] Dietz, M. L. Sep. Sci. Technol. 2006, 41, 2047.
[14] Vidal, S. T.; Correia, M. J.; Marques, M. M.; Ismael, M. R.; Reis, M. T. Sep. Sci. Technol. 2004, 39, 2155.
[15] De los Ríos, A. P.; Lozano, L. J.; Sánchez, S.; Moreno, J.; Godinez, C. J. Chem. Eng. Data 2010, 55, 605.
[16] De los-Ríos, A. P.; Hernandez-Fernandez, F. J.; Alguacil, F. J.; Alguacil, L. J.; Ginestá, A.; García-Díaz, I.; Sánchez-Segado, S.; López, F. A.; Godínez, C. Sep. Sci. Technol. 2012, 97, 150.
[17] Nobuyuki, K.; Yasuhisa, I. Monatsh. Chem. 2007, 138, 1145.
[18] Dai, S.; Ju, Y. H.; Barnes, C. E. J. Chem. Soc. Dalton Trans. 1999, 1201.
[19] Luo, H. M.; Dai, S.; Bonnesen, P. V. Anal. Chem. 2004, 76, 2773.
[20] Luo, H. M.; Dai, S.; Bonnesen, P. V.; Buchanan, A. C.; Holbrey, J. D.; Bridges, N. J.; Rogers, R. D. Anal. Chem. 2004, 76, 3078.
[21] Wei, G. T.; Yang, Z.; Chen, C. J. Anal. Chim. Acta 2003, 488,183.
[22] Li, C. P.; Xin, B. P.; Xu, W. G. J. Dalian Maritime Univ. 2008, 34, 17.
[23] Chen, S.; Sun, H.; Zhong, Y. S. J. Beijing Univ. Technol. 2013, 39, 98. (陈莎, 孙浩, 钟嶷盛, 北京工业大学学报, 2013, 39, 98.)
[24] Hirayama, N.; Deguchic, M.; Kawasumia, H.; Honjo, T. Talanta 2005, 65, 255.
[25] Tsukatani, T.; Katano, H.; Tatsumi, H.; Deguchi , M.; Hirayama, N. Anal. Sci. 2006, 22, 199.
[26] Li, Z. J.; Wei, Q.; Yuan, R.; Zhou, X.; Liu, H. Z.; Shan, H. X.; Song, Q. J. Talanta 2007, 71, 68.
[27] Dadfarnia, S.; Haji-Shabani, A. M.; Bidabadi, M. S.; Ali Jafari, A. J. Hazard. Mater. 2010, 173, 534.
[28] Visser, A. E.; Swatloski, R. P.; Reichert, W. M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J. H.; Rogers, R. D. Environ. Sci. Technol. 2002, 36, 2523.
[29] Papaiconomou, N.; Lee, J. M.; Salminen, J.; Stosch, M.; Prausnitz, J. M. Ind. Eng. Chem. Res. 2008, 47, 5080.
[30] Lee, J. M. Fluid Phase Equilib. 2012, 319, 30.
[31] Ouadi, A.; Gadenne, Hesemann, P.; Moreau-Joel, J. E.; Billard, I.; Gaillard, C.; Mekki, S.; Moutiers, G. Chem. Eur. J. 2006, 12, 3074.
[32] Jitendra, R. H.; Tomislav, F.; Mac-Gillivray, L. R.; Robert, D. S. Inorg. Chem. 2006, 45, 10025.
[33] Fischer, L.; Falta, T.; Koellensperger, G..; Stojanovic, A.; Kogelnig, D.; Galanski, M.; Krachler, R.; Keppler, B. K.; Hann, S. Water Res. 2011, 45, 4601.
[34] Messadi, A.; Mohamadou, A.; Boudesocque, S.; Dupont, L. Sep. Sci. Technol. 2013, 107, 172.
[35] Devereux, M.; Popelier, P.; Mclay, I. M. Phys. Chem. Chem. Phys. 2009, 11, 1595.
[36] Bader, R.; Carroll, M. T.; Cheeseman, J. R.; Chang, C. J. Am. Chem. Soc. 1987, 109, 7968.
[37] Bulat, F. A.; Toro-Labbe, A.; Brinck, T.; Murray, J. S.; Politzer, P. J. Mol. Model. 2010, 16, 1679.
[38] Pinter, B.; Nagels, N.; Herrebout, W. A.; De Proft, F. Chem-Eur. J. 2013, 19, 519.
[39] Parr, R. G.; Yang, W. J. Am. Chem. Soc. 1984, 106, 4049.
[40] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian09 B.01, Gaussian, Inc., Wallingford, CT, 2009.
[41] Wu, W. H.; Lu, Y. X.; Liu, Y. T.; Li, H. Y.; Peng, C. J.; Liu, H. L.; Zhu, W. L. Chem. Phys. Lett. 2013, 582, 49.
[42] Wu, W. H.; Lu, Y. X.; Liu, Y. T.; Peng, C. J.; Liu, H. L. Comput. Theor. Chem. 2014, 1029, 21.
[43] Dong, K.; Song, Y. T.; Liu, X. M.; Cheng, W. G.; Yao, X. Q.; Zhang, S. J. J. Phys. Chem. B 2012, 116, 1007.
[44] Xu, D.; Yang, Q. W.; Su, B. G.; Bao, Z. B.; Ren, Q. L.; Xing, H. B. J. Phys. Chem. B 2014, 118, 1071.
[45] Boys, S. F.; Bernardi, F. Mol. Phys. 1970, 19, 553.
[46] Murray, J. S.; Ranganathan, S.; Politzer, P. J. Org. Chem. 1991, 56, 3734.
[47] Lu, T.; Chen, F. J. Comput. Chem. 2012, 33, 580.
/
| 〈 |
|
〉 |