

Fmoc和Boc双取代赖氨酸环二肽的合成及其凝胶化性能研究
A Study on Synthesis and Gelation Capability of Fmoc and Boc Disubstituted Cyclo(L-Lys-L-Lys)s
Received date: 2015-02-09
Online published: 2015-03-20
Supported by
Project supported by the National Natural Science Foundation of China (No. 21174018).
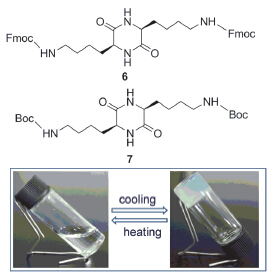
以Boc-Lys(Cbz)-OH为原料首先合成出赖氨酸环二肽, 进而再分别与Fmoc-OSu和(Boc)2O反应得到Fmoc和Boc双取代赖氨酸环二肽. 发现这两种赖氨酸环二肽衍生物在常见醇类、甲苯和氯代溶剂中能形成稳定的热可逆凝胶, 最低凝胶化浓度(MGC)范围为1%~5% (w). 凝胶的储存模量均高于损耗模量, 并且复合粘度在频率扫描范围内呈线性降低趋势. 电镜分析表明, 凝胶因子在不同溶剂中可自组装成微纳米纤维网络结构, 纤维直径越细, 凝胶透明度越高. 红外和荧光光谱分析表明在自组装的过程中氢键主要在甲苯和氯代溶剂中发挥驱动力的作用, π-π堆积主要在醇类溶剂中起作用. 核磁氢谱测试表明来自凝胶因子骨架上酰胺和侧链上氨酯基中的氢键共同参与了凝胶化过程.
宗倩颖 , 耿慧敏 , 王璐 , 叶霖 , 张爱英 , 邵自强 , 冯增国 . Fmoc和Boc双取代赖氨酸环二肽的合成及其凝胶化性能研究[J]. 化学学报, 2015 , 73(5) : 423 -430 . DOI: 10.6023/A15020111
Fmoc and Boc disubstituted cyclo(L-Lys-L-Lys)s were synthesized using Boc-Lys(Cbz)-OH as starting material to prepare lysine cyclic dipeptide followed by the reaction with Fmoc-OSu and (Boc)2O, respectively. Using test tube inversion method, two derivatives were found to form stable organogels in common alcoholic, methylbenzene and chlorinated solvents holding a minimum gelation concentration (MGC) in a range of 1%~5% (w). All the formed organogels exhibited thermal reversibility, which can change into transparent solutions under heating. The TEM and SEM observations of their xerogels showed that these organogelators were self-assembled into 3D network structures, such as nanofibres, nanoribbons or nanosheets. The thinner the fiber diameter was, the more transparent the organogel became. The rheological measurements revealed that the storage modulus of two kinds of gels was higher than the loss modulus, and the complex viscosity was reduced linearly with the increase of scanning frequency. Fluorescence spectroscopy of Fmoc disubstituted cyclo(L-Lys-L-Lys) (6) in methanol and 1,2-dichloroethane showed that the main emission peak of Fmoc red-shifted to 342 and 328 nm respectively, and more multiple emission peaks appeared between 350 nm and 600 nm. FT-IR results of Boc disubstituted cyclo(L-Lys-L-Lys) (7) organogels and its solid sample indicated that not only lysine cyclic dipeptide skeleton itself participated in the self-assembly, but also the hydrogen bondings between the urethane on the side-chain contributed to the organogel formation. The hydrogen bondings played mainly a role in driving the self-assembly process of gelators in methylbenzene and chlorinated solvents, whereas π-π stacking generally acted as a driving force for the self-assembly process in alcoholic solvents. A X-Ray diffraction pattern was obtained from the sample prepared with 7 in methylbenzene, showing reflections at 4.6 and 24.4 Å, attributed to the molecular simulation size and the interstrand distances between β-sheet structures, respectively.

[1] Steed, J. W. Chem. Commun. 2011, 47, 1379.
[2] Suzaki, Y.; Taira, T.; Osakada, K. J. Mater. Chem. 2011, 21, 930.
[3] Hanabusa, K.; Suzuki, M. Polym. J. 2014, 46, 776.
[4] Plekan, O.; Feyer, V.; Ptasinska, S.; Tsud, N.; Prince, K. C. Phys. Chem. Chem. Phys. 2014, 16, 6657.
[5] Xie, Z. G.; Zhang, A. Y.; Ye, L.; Wang, X.; Feng, Z. G. J. Mater. Chem. 2009, 19, 6100.
[6] Adams, D. J.; Butler, M. F.; Frith, W. J.; Kirkland, M.; Mullen, L.; Sanderson, P. Soft Matter. 2009, 5, 1856.
[7] Sadownik, J. W.; Ulijn, R. V. Chem. Commun. 2010, 46, 3481.
[8] Shen, L. Y.; Chen, X. Z.; Yu, H. T. Chin. J. Org. Chem.2009, 29, 321. (沈丽英, 陈肖卓, 于海涛, 有机化学, 2009, 29, 321.)
[9] Fleming, S.; Ulijn, R. V. Chem. Soc. Rev. 2014, 43, 8150.
[10] Fichman, G.; Adler, A. L.; Manohar, S. ACS Nano 2014, 8, 7220.
[11] Yan, X.; Zhu, P.; Li, J. Chem. Soc. Rev. 2010, 39, 1877.
[12] Aggeli, A.; Bell, M.; Boden, N. J. Mater. Chem. 1997, 7, 1135.
[13] Hanabusa, K.; Fukui, H.; Suzuki, M.; Shirai, H. Langmuir 2005, 21, 10383.
[14] Hoshizawa, H.; Minemura, Y.; Yoshikawa, K.; Suzuki, M.; Hanabusa, K. Langmuir 2013, 29, 14666.
[15] Xie, Z. G.; Zhang, A. Y.; Ye, L.; Feng, Z. G. Soft Matter 2009, 5, 1474.
[16] Manchineella, S.; Govindaraju, T. RSC Adv. 2012, 2, 5539.
[17] Xie, Z. G.; Zhang, A. Y.; Ye, L.; Feng, Z. G. Acta Chim. Sinica 2008, 66, 2620. (谢志国, 张爱英, 叶霖, 冯增国, 化学学报, 2008, 66, 2620. )
[18] Ma, L. X. Ph.D. Dissertation, Shandong University, Jinan, 2008. (马丽霞, 博士论文, 山东大学, 济南, 2008.)
[19] Geng, H.; Zong Q.; You J.; Ye L.; Zhang, A. Y.; Feng, Z. G. Chin. J. Appl. Chem. 2015 (in revision) (耿慧敏, 宗倩颖, 尤劼, 叶霖, 张爱英, 冯增国, 应用化学, 2015(修稿中))
[20] Maria, A. M.; Jordi, J. B. Macromol. Chem. Phys. 2006, 207, 615.
[21] Kaur, N.; Zhou, B.; Breitbeil, F.; Hardy, K.; Kraft, K. S.; Trantcheva, I.; Phanstiel, O. Mol. Pharm. 2008, 5, 294.
[22] Skilling, K. J.; Citossi, F.; Bradshaw, T. D.; Ashford, M.; Kellam, B.; Marlow, M. Soft. Matter. 2014, 10, 237.
[23] Yang, Z. M.; Xu, B. J. Mater. Chem. 2007, 17, 2385.
[24] Solsona, M. T.; Miravet, J. F. Chem. Eur. J. 2014, 20, 1023.
[25] Yang, Z. M.; Liang, G. L.; Ma, M. L.; Gao, Y.; Xu, B. Small 2007, 3, 558.
[26] Sadownik, J. W.; Ulijn, R. V. Chem. Commun. 2010, 46, 3481.
[27] Shao, H.; Parquette, J. R. Chem. Commun. 2010, 46, 4285.
[28] Cui, H. G.; Muraoka, T.; Cheetham, A. G.; Stupp, S. I. Nano Lett. 2009, 9, 945.
[29] Cheng, P. N.; Pham, J. D.; Nowick, J. S. J. Am. Chem. Soc.2013, 135, 5477.
/
| 〈 |
|
〉 |