

含羰基有机添加剂对AlCl3-[Emim]Cl电沉积铝的影响
收稿日期: 2014-12-12
网络出版日期: 2015-04-01
基金资助
项目受国家重点基础研究发展计划(No. 2015CB251401), 国家科技支撑计划(No. 2012BAF03B01)和国家自然科学基金项目(No. 21127011, 51274181)资助.
Effects of Organic Additives Containing Carbonyl Group on Electrodeposition of Al from AlCl3-[Emim]Cl Ionic Liquid
Received date: 2014-12-12
Online published: 2015-04-01
Supported by
Project supported by the National Key Basic Research Program of China (No. 2015CB251401), the Key Technologies R&D Program (No. 2012BAF03B01), the Special Funds of the National Natural Science Foundation of China (No. 21127011) and the General Program of National Natural Science Foundation of China (No. 51274181).
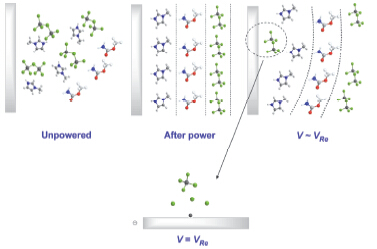
以[Emim]Cl/AlCl3(33.3/66.7 mol%)离子液体为电解质, 选取了丙酮、乙酰胺、乙酸、乙酸甲酯、氨基甲酸甲酯5种含有羰基官能团的有机分子作为添加剂, 讨论其对铝沉积层的影响. 通过CV曲线、SEM、XRD、UV-Vis、NMR等分析, 进一步研究了添加剂对Al沉积层形貌、晶面取向及沉积机理的影响. 结果表明: 氨基甲酸甲酯是一种性能优异的整平添加剂, 45 mmol/L氨基甲酸甲酯的加入明显改善Al产品的光亮度, 得到细致均匀且镜面光亮的Al沉积层. 氨基甲酸甲酯为添加剂时, 在电解液体系中没有形成新的金属络合离子, 不影响电解液中活性铝离子结构; 其羰基碳原子为正电中心在阴极表面吸附, 对Al的电沉积过程产生抑制进而获得整平和光亮效果.
冷明浩 , 陈仕谋 , 张军玲 , 郎海燕 , 康艳红 , 张锁江 . 含羰基有机添加剂对AlCl3-[Emim]Cl电沉积铝的影响[J]. 化学学报, 2015 , 73(5) : 403 -408 . DOI: 10.6023/A14120857
Electrodeposition of Al in ionic liquids for preparation of aluminum products had opened up a new research direction, but the previous studies found that aluminum product from pure ionic liquid electrodeposition was unable to meet the requirements in both uniformity and smoothness. Electrodepostion in pure ionic liquid often results in the deposits with rough surface or even dendritic products after long-time deposition. In this study, five kinds of small organic molecules containing carbonyl as additives were investigated, such as acetone, acetic acid amide, acetic acid, methyl acetate and methyl carbamate. Additives were added to [Emim]Cl/AlCl3(33.3/66.7 mol%) with a concentration of 5 mmol/L, 15 mmol/L, 45 mmol/L. Cyclic voltammetry was studied on a glassy carbon electrode at a scan rate of 100 mV·s-1 and temperature of 323 K. The electrode potential was scanned from the open circuit potential to negative-potential, then retraced to get cyclic voltammetry curve. Among those organic additives, methyl carbamate was proved to be an excellent additive. The brightness of Al can be improved by adding 45 mmol/L methyl carbamate. Smooth, uniform and mirror bright Al deposition would be obtained afterward. Furthermore, the effects of additive for Al deposit on morphology, crystal orientation and Al deposition mechanism by the analysis of galvanostatic deposition, scanning electron microscope (SEM), X-ray diffraction (XRD), ultraviolet-visible spectroscopy (UV-Vis) and nuclear magnetic resonance (NMR) was conjectured. The results showed that methyl carbamate as an additive would make grain refinement more apparent and present more strong Al(200) crystal plane orientation. As an additive, methyl carbamate did not form new metal complex in the electrolytic liquid and did not affect the structure of active aluminum ion in the electrolyte. The positive center of carbonyl carbon atom in methyl carbamate molecular can be easily adsorbed at the cathode surface. This procedure inhibited the process of Al deposition and obtained the smooth and bright effect. Therefore, Al deposition layer of mirror can be obtained, which has an important guiding significance in the selection of ionic liquid additive in electrodeposition system.

Key words: ionic liquids; electrodeposition; carbonyl; methyl carbamate; additives
[1] Chang, J. K.; Chen, S. Y.; Tsai, W. T.; Deng, M. J.; Sun, I. W. Electrochem. Commun. 2007, 9(7), 1602.
[2] Endo, A.; Miyake, M.; Hirato, T. Electrochim. Acta 2014, 137, 470.
[3] NuLi, Y. N.; Du, G. D.; Feng, Z. Z.; Shen, J.-N.; Yang, J. Acta Chim. Sinica 2008, 66, 175. (努丽燕娜, 杜国栋, 冯真真, 沈佳妮, 杨军, 化学学报, 2008, 66, 175.)
[4] NuLi, Y. N.; Yang, J.; Gao, P. F.; Li, Y.; Wang, J. L. Acta Chim. Sinica 2010, 68, 948. (努丽燕娜, 杨军, 高鹏飞, 李云, 王久林, 化学学报, 2010, 68, 948.)
[5] Hurley, F. H.; Wier, T. P. J. Electrochem. Soc. 1951, 85(5), 207.
[6] Liao, Q.; Pitner, W. R.; Stewart, G.; Hussey, C. L.; Stafford, G. R. J. Electrochem. Soc.1997, 144(3), 936.
[7] Lee, J. J.; Bae, I. T.; Scherson, D. A.; Miller, B.; Wheeler, K. A. J. Electrochem. Soc.2000, 147(2), 562.
[8] Liu, Q. X.; El. Abedin, S. Z.; Endres, F. Surf. Coat. Technol. 2006, 201, 1352.
[9] Tang, J. W.; Azumi, K. Electrochim. Acta 2011, 56, 1130.
[10] Zhao, Y. G.; VanderNoot, T. J. Electrochim. Acta 1997, 42(11), 1639.
[11] Chang, J. K.; Chen, S. Y.; Tsaia, W. T.; Deng, M. J.; Sun, I. W. J. Electrochem. Soc.2008, 155(3), C112.
[12] Yue, G. K.; Zhang, S. J.; Zhu, Y. L.; Lu, X. M.; Li, S. C.; Li, Z. X. AIChE J. 2009, 55, 783.
[13] Jiang, T.; Brym, M. J. C.; Dube, G.; Lasia, A.; Brisard, G. M. Surf. Coat. Technol.2006, 201, 10.
[14] Su, C. J.; Hsieh, Y. T.; Chen, C. C. Electrochem. Commun. 2013, 34, 170.
[15] El. Abedin, S. Z.; Moustafa, E. M.; Hempelmann, R.; Natter, H.; Endres, F. Electrochem. Commun. 2005, 7, 1111.
[16] El. Abedin, S. Z.; Moustafa, E. M.; Hempelmann, R.; Natter, H.; Endres, F. ChemPhysChem 2006, 7(11), 1535.
[17] Moustafa, E. M.; El. Abedin, S. Z.; Shkurankov, A.; Zschippang, E.; Saad, A. Y.; Bund, A.; Endres, F. J. Phys. Chem. B 2007, 111, 4693.
[18] Atkin, R.; El. Abedin, S. Z.; Hayes, R.; Gasparotto, L. H. S.; Borisenko, N.; Endres, F. J. Phys. Chem. C 2009, 113, 13266.
[19] Giridhar, P.; El. Abedin, S. Z.; Endres, F. Electrochim. Acta 2012, 70, 21.
[20] Zheng, Y.; Dong, K.; Wang, Q.; Zhang, S. J.; Zhang, Q. Q.; Lu, X. M. Sci. China Chem. 2012, 55(8), 1587.
[21] Zheng, Y.; Zhang, S. J.; Lu, X. M.; Wang, Q.; Zuo, Y.; Liu, L. Chinese J. Chem. Eng.2012, 20(1), 130.
[22] Abbott, A. P.; Qiu, F.; Abood, H. M. A.; Ali, M. R.; Ryder, K. S. Phys. Chem. Chem. Phys. 2010, 12, 1862.
[23] Liu, L.; Lu, X. M.; Cai, Y. J.; Zheng, Y.; Zhang, S. Z. Aust. J. Chem. 2012, 65(11), 1523.
[24] Li, B.; Fan, C. H.; Chen, Y.; Lou, J. W.; Yan, L. G. Electrochim. Acta 2011, 56(16), 5478.
[25] Barchi, L.; Bardi, U.; Caporali, S.; Fantini, M.; Scrivani, A. Prog. Org. Coat. 2010, 68, 120.
[26] Endres, F.; Bukowski, M.; Hempelmann, R.; Natter, H. Angew. Chem. Int. Ed. 2003, 42, 3428.
[27] Zhang, Q. Q.; Wang, Q.; Zhang, S. Z.; Lu, X. M. J. Solid State Electrochem. 2014, 18(1), 257.
[28] Wang, X. M. Ph.D. Dissertation Shandong University of Technology, Zibo, 2010. (王晓铭, 博士论文, 山东理工大学, 淄博, 2010.)
[29] Zhu, Y. L.; Katayama, Y.; Miura, T. Electrochim. Acta 2012, 85(4), 622.
[30] Zhu, Y. L.; Katayama, Y.; Miura, T. Electrochim. Acta 2010, 55(28), 9019.
/
| 〈 |
|
〉 |