

基于Nile Red的线粒体靶向性增强型硫化氢荧光探针及其细胞成像
收稿日期: 2015-03-06
网络出版日期: 2015-05-05
基金资助
项目受国家自然科学基金(No. 21302080)和辽宁省教育厅科研项目(No. L2014010)资助.
A Mitochondrial-Targetable and Turn-On Fluorescent Probe based on Nile Red and Monitoring for H2S in Living Cells
Received date: 2015-03-06
Online published: 2015-05-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 21302080) and Program Funded by Liaoning Province Education Administration (No. L2014010).
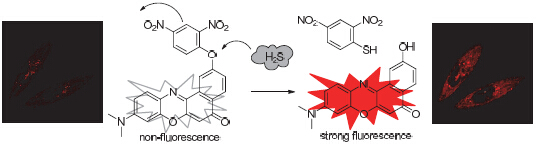
硫化氢作为一类重要的信号分子, 除了在神经系统、炎症应激和心血管等生理系统方面发挥着调控作用之外, 还对线粒体ATP酶的活性和抗氧化应激起着重要的调节作用. 为了实时定量的检测线粒体中的硫化氢, 本文报道了一个可以定位于线粒体并检测外源硫化氢的荧光增强型荧光探针NRS. 探针NRS以尼尔红为母体通过引入吸电子的2,4-二硝基苯结构单元构建了基于光诱导电子转移(PET)机理的荧光探针. 探针NRS表现出快速的硫化氢响应性和较高的硫化氢选择性, 不受其他活性氧、活性氮、阴离子以及金属离子等物种的干扰. 随着硫化钠的加入, 探针NRS的最大吸收峰由565 nm蓝移至550 nm, 溶液由紫色变为红色; 同时在640 nm处的荧光光谱强度不断增强, 当硫化钠的浓度处于32~176 μmol·L-1范围内, 荧光强度与硫化钠的浓度呈现出较好的线性关系. 细胞染色实验表明, 探针NRS能够进入到细胞内部, 具有细胞膜的透过性; 与Rh123进行共定位成像进一步证明探针NRS能够定位于细胞的线粒体中并检测硫化氢.
关键词: 荧光探针; 尼尔红; 线粒体靶向性; 硫化氢; 光诱导电子转移PET
于海波 , 李红玲 , 张新富 , 肖义 , 方沛菊 , 吕春娇 , 侯伟 . 基于Nile Red的线粒体靶向性增强型硫化氢荧光探针及其细胞成像[J]. 化学学报, 2015 , 73(5) : 450 -456 . DOI: 10.6023/A15030158
Despite regulation of some different physiological processes such as nervous system, inflammatory stress and cardiovascular system, hydrogen sulfide (H2S), an important signaling molecule, also can control activity of ATP enzyme and antioxidant stress in mitochondrion of living cells. For real time quantitative detection of H2S, in this paper, a mitochondrial-targetable and turn-on fluorescent probe NRS has been developed and used to monitor exogenous H2S in living cells. Based on the principle of photo-induced electron transfer (PET), 2,4-dinitrobenzen has been introduced into Nile Red moiety in the structure of probe NRS. NRS has been shown fast response and high sensitivity to hydrogen sulfide, without interference from other reactive oxygen species, reactive nitrogen, anions and metal ions species. With addition of sodium sulfide, the maximum absorption wavelength of NRS at 565 nm has blue shifted to 550 nm, and the emission intensity at 640 nm is accordingly increasing. Meanwhile, this solution is turned to purple from red color and visible with naked eye. The emission intensity of NRS is linear function with the sodium sulfide concentration over the range from 32 to 172 祄ol·L-1. The cell-staining experiment indicates that NRS can diffuse across into cells. It is further proof that in colocalization experimentation NRS can be positioned in mitochondria and detecting hydrogen sulfide in living cells.

[1] (a) Liu, Z.; Powers, W.; Murphy, J.; Maghirang, R. J. Anim. Sci. 2014, 92, 1656.
(b) Aneja, V. P.; Blunden, J.; James, K.; Schlesinger, W. H.; Knighton, R.; Gilliam, W.; Jennings, G.; Niyogi, D.; Cole, S. J. Environ. Qual. 2008, 37(2), 515.
(c) Ni, J. Q.; Heber, A. J.; Sutton, A. L.; Kelly, D. T.; Patterson, J. A.; Kim, S. T. Sci. Total. Environ. 2010, 408(23), 5917.
[2] (a) Patni, N. K.; Clarke, S. P. Appl. Eng. Agric. 1991, 7, 478
(b) Wang, K.; Huang, D.; Ying, H.; Luo H. Biosyst. Eng. 2014, 122, 23.
[3] Ankersmita, H. A.; Tennentb, N. H.; Watts, S. F. Atmos. Environ. 2005, 39, 695.
[4] (a) van Loon, G. W.; Duffy, S. J. Environmental Chemistry: a Global Perspective, 2nd ed., Oxford University Press, 2005.
(b) Nowicki, P.; Skibiszewska, P.; Pietrzak R. Chem. Eng. J. 2014, 248, 208.
[5] (a) Whiteman, M.; Armstrong, J. S.; Chu, S. H.; Jia, L. S.; Wong, B. S.; Cheung, N. S.; Halliwell, B.; Moore, P. K. J. Neurochem. 2004, 90, 765.
(b) Kimura, H. Mol. Neurobiol. 2002, 26, 13.
[6] (a) Li, L.; Bhatia, M.; Zhu, Y. Z.; Zhu, Y. C.; Ramnath, R. D.; Wang, Z. J.; Anuar, F. B. M.; Whiteman, M.; Salto-Tellez, M.; Moore, P. K. FASEB J. 2005, 19, 1196.
(b) Zanardo, R. C.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J. L. FASEB J. 2006, 20, 2118.
(c) Dufton, N.; Natividad, J.; Verdu, E. F.; Wallace, J. L. Sci. Rep. 2012, 2, 499.
[7] Wang, R. Kidney Int. 2009, 76, 700.
[8] (a) Gadalla, M. M.; Snyder, S. H. J. Neurochem. 2010, 113, 14.
(b) Li, L.; Rose, P.; Moore, P. K. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169.
[9] (a) Eto, K.; Asada, T.; Arima, K.; Makifuchi, T.; Kimura, H. Biochem. Biophys. Res. Commun. 2002, 293, 1485.
(b) Szabo, C. Nat. Rev. Drug Discov. 2007, 6, 917.
[10] (a) Ishigami, M.; Hiraki, K.; Umemura, K.; Ogasawara, Y.; Ishii, K.; Kimura, H. Antioxid. Redox Sign. 2009, 11, 205.
(b) Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. Antioxid. Redox Sign. 2009, 11, 703.
[11] (a) Cooper, C. E.; Brown, G. C. J. Bioenergy Biomembr. 2008, 40, 533.
(b) Hildebrandt, T. M.; Grieshaber, M. FEBS J. 2008, 275, 3352.
[12] Sun, Y.; Zhang, S. Q.; Jin, H. F.; Tang, C. S.; Du, J. B. Chin. J. Cardiol. 2009, 2, 161. (孙燕, 张素清, 金红芳, 唐朝枢, 杜军保, 中华心血管病杂志, 2009, 2, 161.)
[13] (a) Gu, X.; Liu, C.; Zhu, Y.-C.; Zhu, Y.-Z. Tetrahedron Lett. 2011, 52, 5000.
(b) Fischer, E. Chem. Ber. 1983, 26, 2234.
(c) Hughes, M. N. Free Radical Biol. Med. 2009, 47, 1346.
[14] (a) Lawrence, N. S.; Deo R. P.; Wang, J. Anal. Chim. Acta 2004, 517, 131;
(b) Lawrence, N. S.; Davis, J.; Jiang, L.; Jones, T. G. J.; Davies, S. N.; Compton, R. G. Electroanalysis 2000, 12, 1453.
[15] (a) Levitt, M. D.; Abdel-Rehim, M. S. J. Antioxid. Redox Sign. 2011, 15, 373.
(b) Kolluru, G. K.; Shen, X.; Bir, S. C.; Kevil, C. G. Nitric Oxide 2013, 35, 5.
[16] (a) Gao, M.; Yu, F.; Chen, L. Prog. Chem. 2014, 26(6), 1065. (高敏, 于法标, 陈令新, 化学进展, 2014, 26(6), 1065);
(b) Liu, C.; Ma, X.; Wei, G.; Du, Y. Environ. Chem. 2014, 10(33), 1672. (刘春霞, 马兴, 魏国华, 杜宇国, 环境化学, 2014, 10(33), 1672).
(c) Cao, X.; Lin, W.; Zheng, K.; He, L. Chem. Commun. 2012, 48(85), 10529.
[17] (a) Xu, Z.; Xu, L.; Zhou, J.; Xu, Y.; Zhu, W.; Qian, X. Chem. Commun. 2012, 48(88), 10871.
(b) Wan, Q.; Song, Y.; Li, Z.; Gao, X.; Ma, H. Chem. Commun. 2013, 49(5), 502.
(c) Zhao, Y.; Zhu, X.; Kan, H.; Wang, W.; Zhu, B.; Du, B.; Zhang, X. Analyst 2012, 137(23), 5576.
(d) Chen, Y.; Zhu, C.; Yang, Z.; Chen, J.; He, Y.; Jiao, Y.; He, W.; Qiu, L.; Cen, J.; Guo, Z. Angew. Chem. Int. Ed. 2013, 52(6), 1688.
(e) Yu, F.; Han, X.; Chen, L. Chem. Commun. 2014, 50(82), 12234.
(f) Wang, R.; Yu, F.; Chen, L.; Chen, H.; Wang, L.; Zhang, W. Chem. Commun. 2012, 48(96), 11757.
(g) Yu, F.; Li, P.; Song, P.; Wang, B.; Zhao, J.; Han, K. Chem. Commun. 2012, 48(23), 2852.
[18] (a) Das, S. K.; Lim, C. S.; Yang, S. Y.; Han, J. H.; Cho, B. R. Chem. Commun. 2012, 48, 8395.(b) Liu, X. L.; Du, X. J.; Dai, C. G.; Song, Q. H. J. Org. Chem. 2014, 79(20), 9481.
[19] (a) Mineno, T.; Ueno, T.; Urano, Y.; Kojima, H.; Nagano, T. Org. Lett. 2006, 8(26), 5963.
(b) Fujikawa, Y.; Urano, Y.; Komatsu, T.; Hanaoka, K.; Kojima, H.; Terai, T.; Inoue, H.; Nagano, T. J. Am. Chem. Soc. 2008, 130(44), 14533.
[20] Takahashi, A.; Zhang, Y.; Centonze, E.; Herman, B. Biotechniques 2001, 30(4), 804.
/
| 〈 |
|
〉 |