

铜促进下腙的氧化胺化反应:1H-吲唑和1H-吡唑的合成
收稿日期: 2015-04-16
网络出版日期: 2015-06-15
基金资助
项目受国家自然科学基金(Nos.21272149,21302123),上海市教委科研创新重点项目(No.14ZZ094)以及上海市自然科学基金(No.13ZR1416400)资助.
Copper-Promoted Oxidative C-H Bond Amination of Hydrazones:Synthesis of 1H-Indazoles and 1H-Pyrazoles
Received date: 2015-04-16
Online published: 2015-06-15
Supported by
Project supported by the National Natural Science Foundation of China(Nos. 21272149, 21302123), Innovation Program of Shanghai Municipal Education Commission(No. 14ZZ094) and Science and Technology Commission of Shanghai Municipality(No. 13ZR1416400).
丁正伟 , 谭启涛 , 刘秉新 , 张可 , 许斌 . 铜促进下腙的氧化胺化反应:1H-吲唑和1H-吡唑的合成[J]. 化学学报, 2015 , 73(12) : 1302 -1306 . DOI: 10.6023/A15040263
An efficient copper-promoted C(sp2)-H bond amination was developed to afford 1H-indazoles and 1H-pyrazoles in moderate to excellent yields from easily accessible hydrazones. This process tolerated a variety of functional groups and afforded the corresponding 1H-indazoles and 1H-pyrazoles under mild conditions.
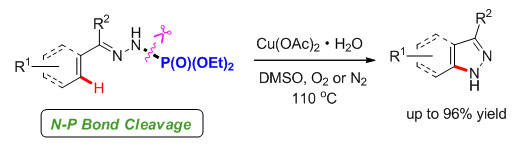
Key words: C-H activation; amination; indazole; nitrogen heterocycles; pyrazole
[1] (a) Joule, J. A.; Mills, K. Heterocyclic Chemistry, 4th ed., Blackwell Science, Oxford, 2000.
(b) Butler, M. S. J. Nat. Prod. 2004, 67, 2141.
(c) Zhao, J.; Zhang, Q. Acta Chim. Sinica 2015, 73, 1235.(赵金钵, 张前, 化学学报, 2015, 73, 1235.)
[2] Fu, X.; Chen, Y.; Yang, Y.; He, S.; Shen, C.; Wan, R. Chin. J. Org. Chem. 2014, 34, 2090.(傅晓东, 陈月, 杨阳, 贺书泽, 沈陈, 万嵘, 有机化学, 2014, 34, 2090.)
[3] Keppler, B. K.; Hartmann, M. Met.-Based Drugs 1994, 1, 145.
[4] Sun, J. H.; Teleha, C. A.; Yan, J. S.; Rodgers, J. D.; Nugiel, D. A. J. Org. Chem. 1997, 62, 5627.
[5] De Lena, M.; Lorusso, V.; Latorre, A.; Fanizza, G.; Gargano, G.; Caporusso, L.; Guida, M.; Catino, A.; Crucitta, E.; Sambiasi, D.; Mazzei, A. Eur. J. Cancer 2001, 37, 364.
[6] Lee, F. Y.; Lien, J. C.; Huang, L. J.; Huang, T. M.; Tsai, S. C.; Teng, C. M.; Wu, C. C.; Cheng, F. C.; Kuo, S. C. J. Med. Chem. 2001, 44, 3746.
[7] For selected reviews, see:(a) Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. Chem. Rev. 2011, 111, 6984.
(b) Schmidt, A.; Dreger, A. Curr. Org. Chem. 2011, 15, 1423.
(c) Ma, S.; Zhong, Y.; Wang, S.; Xu, Z.; Chang, M.; Wang, R. Acta Chim. Sinica 2014, 72, 825.(马世雄, 钟源, 王守磊, 许兆青, 常民, 王锐, 化学学报, 2014, 72, 825.)
[8] (a) Stadlbauer, W. Sci. Synth. 2002, 12, 227.
(b) Jacobson, P.; Huber, L. Ber. Dtsch. Chem. Ges. 1908, 41, 660.
(c) Rüchardt, C.; Hassmann, V. Leibigs Ann. Chem. 1980, 908.
(d) Yoshida, T.; Matsuura, N.; Yamamoto, K.; Doi, M.; Shimada, K.; Morie, T.; Kato, S. Heterocycles 1996, 43, 2701.
[9] (a) Lebedev, A. Y.; Khartulyari, A. S.; Voskoboynikov, A. Z. J. Org. Chem. 2005, 70, 596.
(b) Inamoto, K.; Katsuno, M.; Yoshino, T.; Arai, Y.; Hiroya, K.; Sakamoto, T. Tetrahedron 2007, 63, 2695.
(c) Liu, R.; Zhu, Y.; Qin, L.; Ji, S. Synth. Commun. 2008, 38, 249.
(d) Thomé, I.; Besson, C.; Kleine, T.; Bolm, C. Angew. Chem., Int. Ed. 2013, 52, 7509.
[10] (a) Jin, T.; Yamamoto, Y. Angew. Chem., Int. Ed. 2007, 46, 3323.
(b) Liu, Z.; Shi, F.; Martinez, P. D. G.; Raminelli, C.; Larock, R. C. J. Org. Chem. 2008, 73, 219.
(c) Li, P.; Zhao, J.; Wu, C.; Larock, R. C.; Shi, F. Org. Lett. 2011, 13, 3340.
(d) Li, P.; Wu, C.; Zhao, J.; Rogness, D. C.; Shi, F. J. Org. Chem. 2012, 77, 3149.
(e) Spiteri, C.; Keeling, S.; Moses, J. E. Org. Lett. 2010, 12, 3368.
[11] (a) Nakano, Y.; Hamaguchi, M.; Nagai, T. J. Org. Chem. 1989, 54, 5912.
(b) Foti, F.; Grassi, G.; Risitano, F. Tetrahedron Lett. 1999, 40, 2605.
(c) Huang, Y. R.; Katzenellenbogen, J. A. Org. Lett. 2000, 2, 2833.
(d) Katritzky, A. R.; Wang, M.; Zhang, S.; Voronkov, M. V.; Steel, P. J. J. Org. Chem. 2001, 66, 6787.
(e) Baldwin, J. E.; Pritchard, G. J.; Rathmell, R. E. J. Chem. Soc., Perkin Trans. 1 2001, 2906.
(f) Grotjahn, D. B.; Van, S.; Combs, D.; Lev, D. A.; Schneider, C.; Rideout, M.; Meyer, C.; Hernandez, G.; Mejorado, L. J. Org. Chem. 2002, 67, 9200.
(g) Aggarwal, V. K.; Vicente, J.; Bonnert, R. V. J. Org. Chem. 2003, 68, 538;
(h) Adamo, M. F. A.; Adlington, R. M.; Baldwin, J. E.; Pritchard, G. J.; Rathmell, R. E. Tetrahedron 2003, 59, 2197.
(i) Heller, S. T.; Natarajan, S. R. Org. Lett. 2006, 8, 2675.
(j) Dirat, O.; Clipson, A.; Elliott, J. M.; Garrett, S.; Jones, A. B.; Reader, M.; Shaw, D. Tetrahedron Lett. 2006, 47, 1729.
(k) Smith, C. D.; Tchabanenko, K.; Adlington, R. M.; Baldwin, J. E. Tetrahedron Lett. 2006, 47, 3209.
(l) Fustero, S.; Román, R.; Sanz-Cervera, J. F.; Simón-Fuentes, A.; Cuñat, A. C.; Villanova, S.; Murguía, M. J. Org. Chem. 2008, 73, 3523.
(m) Fustero, S.; Román, R.; Sanz-Cervera, J. F.; Simón-Fuentes, A.; Bueno, J.; Villanova, S. J. Org. Chem. 2008, 73, 8545.
(n) Liu, H. L.; Jiang, H. F.; Zhang, M.; Yao, W. J.; Zhu, Q. H.; Tang, Z. Tetrahedron Lett. 2008, 49, 3805.
[12] (a) Hao, L.; Hong, J. J.; Zhu, J.; Zhan, Z. P. Chem. Eur. J. 2013, 19, 5715.
(b) Zhu, Y.; Lu, W.-T.; Sun, H. C.; Zhan, Z. P. Org. Lett. 2013, 15, 4146.
(c) Zhang, G.; Ni, H.; Chen, W.; Shao, J.; Liu, H.; Chen, B.; Yu, Y. Org. Lett. 2013, 15, 5967.
(d) Kong, Y.; Tang, M.; Wang, Y. Org. Lett. 2014, 16, 576.
(e) Schneider, Y.; Prévost, J.; Gobin, M.; Legault, C. Y. Org. Lett. 2014, 16, 596.
[13] For recently reviews, see:(a) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
(b) Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068.
(c) Zhang, C.; Tang, C.; Jiao, N. Chem. Soc. Rev. 2012, 41, 4364.
(d) Bariwal, J.; Eycken, E. V. Chem. Soc. Rev. 2013, 42, 9283.
(e) Louillat, M. L.; Patureau, F. W. Chem. Soc. Rev. 2014, 43, 901.
[14] For selected examples, see:(a) Brasche, G.; Buchwald, S. L. Angew. Chem., Int. Ed. 2008, 47, 1932.
(b) Neumann, J. J.; Rakshit, S.; Dröge, T.; Glorius, F. Angew. Chem., Int. Ed. 2009, 48, 6892.
(c) Cho, S. H.; Yoon, J.; Chang, S. J. Am. Chem. Soc. 2011, 133, 5996.
(d) He, G.; Zhao, Y.; Zhang, S.; Lu, C.; Chen, G. J. Am. Chem. Soc. 2012, 134, 3.
(e) Wang, X.; Jin, Y.; Zhao, Y.; Fu, H. Org. Lett. 2012, 14, 452.
(f) Tran, L. D.; Roane, J.; Daugulis, O. Angew. Chem., Int. Ed. 2013, 52, 6043.
(g) Kim, H.; Shin, K.; Chang, S. J. Am. Chem. Soc. 2014, 136, 5904.
[15] (a) Inamoto, K.; Saito, T.; Katsuno, M.; Sakamoto, T.; Hiroya, K. Org. Lett. 2007, 9, 2931.
(b) Hu, J.; Chen, S.; Sun, Y.; Yang, J.; Rao, Y. Org. Lett. 2012, 14, 5030.
(c) Zhang, T.; Bao, W. J. Org. Chem. 2013, 78, 1317.
(d) Li, X.; He, L.; Chen, H.; Wu, W.; Jiang, H. J. Org. Chem. 2013, 78, 3636.
(e) Yu, D. G.; Suri, M.; Glorius, F. J. Am. Chem. Soc. 2013, 135, 8802.
(f) Han, S.; Shin, Y.; Sharma, S.; Mashra, N. K.; Park, J.; Kim, M.; Kim, M.; Jang, J.; Kim, I. S. Org. Lett. 2014, 16, 2494.
[16] (a) Li, G.; Ding, Z.; Xu, B. Org. Lett. 2012, 14, 5338.
(b) Liu, W.; Hong, X.; Xu, B. Synthesis 2013, 45, 2137.
[17] (a) Huang, X.; Xu, S.; Tan, Q.; Gao, M.; Li, M.; Xu, B. Chem. Commun. 2014, 50, 1465.
(b) Wang, H.; Yu, Y.; Hong, X.; Tan, Q.; Xu, B. J. Org. Chem. 2014, 79, 3279.
(c) Fang, T.; Tan, Q.; Ding, Z.; Liu, B.; Xu, B. Org. Lett. 2014, 16, 2342.
(d) Sun, J.; Tan, Q.; Yang, W.; Liu, B.; Xu, B. Adv. Synth. Catal. 2014, 356, 388.
(e) Qian, G.; Liu, B.; Tan, Q.; Zhang, S.; Xu, B. Eur. J. Org. Chem. 2014, 4837.
(f) Ding, Z.; Tan, Q.; Gao, M.; Xu, B. Org. Biomol. Chem. 2015, 13, 4642.
[18] (a) Henke, B. R.; Willson, T. M.; Sugg, E. E.; Croom, D. K.; Jr. Doughety, R. W.; Queen, K. L.; Birkemo, L. S.; Ervin, G. N.; Grizzle, M. K.; Johnson, M. F.; James, M. K. J. Med. Chem. 1996, 39, 2655.
(b) Sun, J. H.; Teleha, C. A.; Yan, J. S.; Rodgers, J. D.; Nugiel, D. A. J. Org. Chem. 1997, 62, 5627.
(c) Dymock, B. W.; Barril, X.; Brough, P. A.; Cansfield, J. E.; Massey, A.; McDonald, E.; Hubbard, R. E.; Surgenor, A.; Roughley, S. D.; Webb, P.; Workman, P.; Wright, L.; Drysdale, M. J. J. Med. Chem. 2005, 48, 4212.
(d) Collins, I.; Workman, P. Nat. Chem. Biol. 2006, 2, 689.
(e) Zanaletti, R.; Bettinetti, L.; Castaldo, C.; Cocconcelli, G.; Comery, T.; Dunlop, J.; Gaviraghi, G.; Ghiron, C.; Haydar, S. N.; Jow, F.; Maccari, L.; Micco, I.; Nencini, A.; Scali, C.; Turlizzi, E.; Valacchi, M. J. Med. Chem. 2012, 55, 4806.
[19] Qi, Q.; Shen, Q.; Lu, L. J. Am. Chem. Soc. 2012, 134, 6548.
[20] Hu, J.; Xu, H.; Nie, P.; Xie, X.; Nie, Z.; Rao, Y. Chem. Eur. J. 2014, 20, 3932.
[21] Wang, H.; Wang, Y.; Peng, C.; Zhang, J.; Zhu, Q. J. Am. Chem. Soc. 2010, 132, 13217.
[22] Diethyl phosphate has been reported to be an excellent leaving group and the N-P bond of N-diethoxyphosphoryl hydrazone was labile under basic conditions, see:(a) Wadsworth, W. S.; Emmons, W. D. J. Org. Chem. 1967, 32, 1279.
(b) Koziara, A.; Turski, K.; Zwierzak, A. Synthesis 1986, 298.
(c) Galeta, J.; Man, S.; Bouillon, J. P.; Potá?ek, M. Eur. J. Org. Chem. 2011, 392.
(d) Wen, J.; Yang, C. T.; Jiang, T.; Hu, S.; Yang, T. Z.; Wang, X. L. Org. Lett. 2014, 16, 2398.
[23] A signal at δ-4.81 was observed in the 31P NMR of the reaction mixture after the disappearance of 1a, which is assignable to be diethyl hydrogen phosphate when compared with the 31P NMR spectrum of the authentic sample(δ-4.67). For details, see:Figure S1 and S2 in the supporting information.
/
| 〈 |
|
〉 |