

酰胺基团导向普通烷烃C-H键的选择性功能化
Selective Functionalization of Normal Alkyl C-H Bonds Using Amides as Directing Groups
Received date: 2015-04-24
Online published: 2015-06-15
Supported by
Project supported by the National Natural Science Foundation of China(No. 21372153).
周励宏 , 陆文军 . 酰胺基团导向普通烷烃C-H键的选择性功能化[J]. 化学学报, 2015 , 73(12) : 1250 -1274 . DOI: 10.6023/A15040278
Normal alkyl C-H bonds refer to those sp3 C-H bonds not linking with any carbon functional groups or hetero-atoms at their adjacent positions. They are fundamental chemical bonds, which are widespread both in raw materials such as petroleum oil, natural gas etc. for chemical industries and in various kinds of intermediates for chemical syntheses. Selective transformation of these ubiquitous alkyl C-H bonds into new carbon-carbon, carbon-nitrogen, or carbon-oxygen bonds is an ideal method in construction of molecular skeletons, extension of carbon-chains and introduction of functional groups, which is urgently desired in either large-scale production or laboratory synthesis. However, these normal sp3 C-H bonds are extremely chemical inert, due to their high bond dissociation energy and low acid dissociation constant. Thus, they are not used directly and conveniently as other available functional groups in synthetic chemistry. Since the beginning of this century, the functionalization of normal alkyl C-H bonds has been developed rapidly through C-H activation which is to cleave these inert C-H bonds with the assistance of some special directing groups by use of catalytic transition-metal complexes under mild conditions. Amides as one of the effective directing groups, which are prevalent in natural products and pharmaceuticals, has also received particular attention. A series of novel reactions using various amides as directing groups to produce carbon-carbon and carbon-heteroatom bonds, including alkyl-aryl, alkyl-alkenyl, alkyl-carbonyl, alkyl-alkynyl, alkyl-alkyl linkages and carbon-nitrogen, carbon-oxygen, carbon-chlorine, carbon-bromine, carbon-boron, carbon-sulfur, carbon-selenium bonds, etc. have been established, and some of them have been applied to the practical synthesis already. This review describes the development on the functionalization of normal alkyl C-H bonds according to the formed bonds, reaction substrates, and chronological order. It is emphasized that the structures of amide groups, properties of transition metals, regional and stereo-selectivities in C-H activations, reaction mechanisms, subsequent transformations and their synthetic applications, etc.
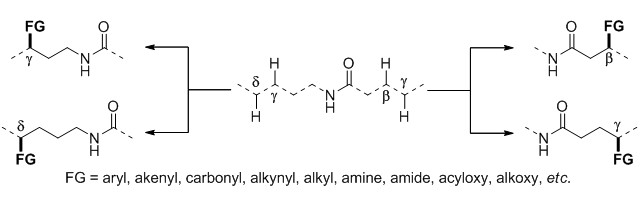
Key words: C-H activation; alkane; functionalization; amide; directing group
[1] (a) Shilov, A. E.; Shul'pin, G. B. Chem. Rev. 1997, 97, 2879.
(b) Labinger, J. A.; Bercaw, J. E. Nature 2002, 417, 507.
(c) Bergman, R. G. Nature 2007, 446, 391.
(d) Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem. Int. Ed. 2009, 48 5094.
(e) Daugulis, O.; Do, H.-Q.; Shabashov, D. Acc. Chem. Res. 2009, 42, 1074.
(f) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
(g) Wasa, M.; Engle, K. M.; Yu, J.-Q. Isr. J. Chem. 2010, 50, 605.
(h) Gutekunst, W. R.; Baran, P. S.; Chem. Soc. Rev. 2011, 40, 1976.
(i) Yagamuchi, J.; Yamaguchi, A. D.; Itami, K. Angew. Chem. Int. Ed. 2012, 51 8960.
(j) Wencel-Delord, J.; Glorius, F. Nat. Chem. 2013, 5, 369.
(k) Rouquet, G.; Chatani, N. Angew. Chem. Int. Ed. 2013, 52 11726.
(l) Zhang, W.; Zhang, J.; Liu, Y. Chin. J. Org. Chem. 2014, 34, 36.(张巍, 张家慧, 刘运奎, 有机化学, 2014, 34, 36.)
(m) Shi, G.; Zhang, Y. Adv. Synth. Catal. 2014, 356, 1419.
(n) Zhang, Q.; Chen, K.; Shi, B.-F. Synlett 2014, 25, 1941.
(o) Zhang, B.; Guan, H.; Liu, B.; Shi, B. Chin. J. Org. Chem. 2014, 34, 1487.(张博, 管晗曦, 刘斌, 史炳峰, 有机化学, 2014, 34, 1487.)
(p) Noisier, A. F. M.; Brimble, M. A. Chem. Rev. 2014, 114, 8775.
(q) Huang, Z.; Dong, G. Tetrahedron Lett. 2014, 55, 5869.
(r) Liu, B.; Shi, B.-F. Tetrahedron Lett. 2015, 56, 15.
(s) Qiu, G.; Wu, J. Org. Chem. Front. 2015, 2, 169.
(t) Chen, T.; Zhang, M. Chin. J. Org. Chem. 2015, 35, 813.(陈天保, 章明, 有机化学, 2015, 35, 813.)
(u) Tan, M.; Gu, Y.; Luo,
X.; Zhang, P. Chin. J. Org. Chem. 2015, 35, 781.(谭明雄, 顾运琼, 罗旭键, 张培, 有机化学, 2015, 35, 781.)
(v) Krylov, I. B.; Vil', V. A.; Terent'ev, A. O. Beilstein J. Org. Chem. 2015, 11, 92.
(w) Wang, M.; Wang, Z.; Shang, M.; Dai, H. Chin. J. Org. Chem. 2015, 35, 570.(王明明, 王子潇, 商明, 戴辉雄, 有机化学, 2015, 35, 570.)
(x) Yang, L.; Huang, H. Chem. Rev. 2015, 115, 3468.
[2] (a) Zaitsev, V. G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 127, 13154.
(b) Reddy, B. V. S.; Reddy, L. R.; Corey, E. J. Org. Lett. 2006, 8, 3391.
(c) Wasa, M.; Engle, K. M.; Yu, J.-Q. J. Am. Chem. Soc. 2009, 131, 9886.
(d) Feng, Y.; Chen, G. Angew. Chem. Int. Ed. 2010, 49, 958.
(e) Li, B. T. Y.; White, J. M.; Hutton, C. A. Aust. J. Chem. 2010, 63, 438.
(f) Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2010, 132, 3965.
(g) Feng, Y.; Wang, Y.; Landgraf, B.; Liu, S.; Chen, G. Org. Lett. 2010, 12, 3414.
(h) Gutekunst, W. R.; Baran, P. S. J. Am. Chem. Soc. 2011, 133, 19076.
(i) Tran, L. D.; Daugulis, O. Angew. Chem. Int. Ed. 2012, 51, 5188.
(j) Gutekunst, W. R.; Gianatassio, R.; Baran, P. S. Angew. Chem. Int. Ed. 2012, 51, 7507.
(k) Wasa, M.; Chan, K. S. L.; Zhang, X.-G.; He, J.; Miura, M.; Yu, J.-Q. J. Am. Chem. Soc. 2012, 134, 18570.
(l) Zhang, Q.; Chen, K.; Rao, W.; Zhang, Y.; Chen, F.-J.; Shi, B.-F. Angew. Chem. Int. Ed. 2013, 52, 13588.
(m) Gutekunst, W. R.; Baran, P. S. J. Org. Chem. 2014, 79, 2430.
(n) Aihara, Y.; Chatani, N. J. Am. Chem. Soc. 2014, 136, 898.
(o) Li, M.; Dong, J.; Huang, X.; Li, K.; Wu, Q.; Song, F.; You, J. Chem. Commun. 2014, 50, 3944.
(p) He, J.; Li, S.; Deng, Y.; Fu, H.; Laforteza, B. N.; Spangler, J. E.; Homs, A.; Yu, J.-Q. Science, 2014, 343, 1216.
(q) Deng, Y.; Gong, W.; He, J.; Yu, J.-Q. Angew. Chem. Int. Ed. 2014, 53, 6692.
(r) Zhang, Q.; Yin, X.-S.; Zhao, S.; Fang, S.-L.; Shi, B.-F. Chem. Commun. 2014, 50, 8353.
(s) Wang, B.; Nack, W. A.; He, G.; Zhang, S.-Y.; Chen, G. Chem. Sci. 2014, 5, 3952.
(t) Affron, D. P.; Davis, O. A.; Bull, J. A. Org. Lett. 2014, 16, 4956.
(u) Feng, R.; Wang, B.; Liu, Y.; Liu, Z.; Zhang, Y. Eur. J. Org. Chem. 2015, 142.
(v) Gong, W.; Zhang, G.; Liu, T.; Giri, R.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 16940.
(w) He, G.; Zhang, S.-Y.; Nack, W. A.; Pearson, R.; Rabb-Lynch, J.; Chen, G. Org. Lett. 2014, 16, 6488.
(x) Chen, G.; Shigenari, T.; Jain, P.; Zhang, Z.; Jin, Z.; He, J.; Li, S.; Mapelli, C.; Miller, M. M.; Poss, M. A.; Scola, P. M.; Yeung, K.-S.; Yu, J.-Q. J. Am. Chem. Soc. 2015, 137, 3338.
(y) Chen, K.; Li, Z.-W.; Shen, P.-X.; Zhao, H.-W.; Shi, Z.-J. Chem. Eur. J. 2015, 21, 7389.
[3] (a) He, G.; Chen, G. Angew. Chem. Int. Ed. 2011, 50, 5192.
(b) Nadres, E. T.; Santos, G. I. F.; Shabashov, D.; Daugulis, O. J. Org. Chem. 2013, 78, 9689.
(c) Fan, M.; Ma, D. Angew. Chem. Int. Ed. 2013, 52, 12152.
(d) Cui, W.; Chen, S.; Wu, J.-Q.; Zhao, X.; Hu, W.; Wang, H. Org. Lett. 2014, 16, 4288.
[4] (a) Yan, J.-X.; Li, H.; Liu, X.-W.; Shi, J.-L.; Wang, X.; Shi, Z.-J. Angew. Chem. Int. Ed. 2014, 53, 4945.
(b) Wei, Y.; Tang, H.; Cong, X.; Rao, B.; Wu, C.; Zeng, X. Org. Lett. 2014, 16, 2248.
[5] (a) Wang, D.-H.; Wasa, M.; Giri, R.; Yu, J.-Q. J. Am. Chem. Soc. 2008, 130, 7190.
(b) Wasa, M.; Engle, K. M.; Lin, D. W.; Yoo, E. J.; Yu, J.-Q. J. Am. Chem. Soc. 2011, 133, 19598.
(c) Xiao, K.-J.; Lin, D. W.; Miura, M.; Zhu, R.-Y.; Gong, W.; Wasa, M.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 8138.
[6] (a) Pan, F.; Shen, P.-X.; Zhang, L.-S.; Wang, X.; Shi, Z.-J. Org. Lett. 2013, 15, 4758;
(b) Iyanaga, M.; Aihara, Y.; Chatani, N. J. Org. Chem. 2014, 79, 11933.
(c) Gou, Q.; Zhang, Z.-F.; Liu, Z.-C.; Qin, J. J. Org. Chem. 2015, 80, 3176.
[7] (a) Shang, R.; Ilies, L.; Matsumoto, A.; Nakamura, E. J. Am. Chem. Soc. 2013, 135, 6030.
(b) Gu, Q.; Al Mamari, H. H.; Graczyk, K.; Diers, E.; Ackermann, L. Angew. Chem. Int. Ed. 2014, 53, 3868.
[8] He, J.; Takise, R.; Fu, H.; Yu, J.-Q. J. Am. Chem. Soc. 2015, 137, 4618.
[9] Liegault, B.; Fagnou, K.; Organometallics 2008, 27, 4841.
[10] (a) Wasa, M.; Engle, K. M.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 3680.
(b) Li, S.; Chen, G.; Feng, C.-G.; Gong, W. Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 5267.
[11] (a) He, G.; Chen, G. Angew. Chem. Int. Ed. 2011, 50, 5192.
(b)
Wang, B.; Lu, C.; Zhang, S.-Y.; He, G.; Nack, W. A.; Chen, G. Org. Lett. 2014, 16, 6260.
(c) Shan, G.; Huang, G.; Rao, Y. Org. Bi-omol. Chem. 2015, 13, 697.
(d) Liu, Y.-J.; Zhang, Z.-Z.; Yan, S.-Y.; Liu, Y.-H.; Shi, B.-F. Chem. Commun. 2015, 51, 7899.
[12] (a) Giri, R.; Maugel, N.; Foxman, B. M.; Yu, J.-Q. Organometallics, 2008, 27, 1667.
(b) Ma, Y.; Li, W.; Yu, B. Acta Chim. Sinica 2013, 71, 541.(马玉勇, 李微, 俞飚, 化学学报, 2013, 71, 541.)
[13] (a) Yoo, E. J.; Wasa, M.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 17380.
(b) Hasegawa, N.; Charra, V.; Inoue, S.; Fukumoto, Y.; Chatani, N. J. Am. Chem. Soc. 2011, 133, 8070.
(c) Hasegawa, N.; Shibata, K.; Charra, V.; Inoue, S.; Fukumoto, Y.; Chatani, N. Tetrahedron 2013, 69, 4466.
[14] Wu, X.; Zhao, Y.; Ge, H. J. Am. Chem. Soc. 2015, 137, 4924.
[15] (a) Ano, Y.; Tobisu, M.; Chatani, N. J. Am. Chem. Soc. 2011, 133, 12984.
(b) He, J.; Wasa, M.; Chan, K. S. L.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 3387.
(c) Wang, B.; He, G.; Chen, G. Science China Chem. 2015, doi:10.1007/s11426-015-5392-z.
[16] (a) Zhang, S.-Y.; He, G.; Nack, W. A.; Zhao, Y.; Li, Q.; Chen, G. J. Am. Chem. Soc. 2013, 135, 2124.
(b) Chen, K.; Hu, F.; Zhang, S.-Q.; Shi, B.-F. Chem. Sci. 2013, 4, 3906.
(c) Zhang, S.-Y.; Li, Q.; He, G.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2013, 135, 12135.
(d) Wu, X.; Zhao, Y.; Ge, H. J. Am. Chem. Soc. 2014, 136, 1789.
(e) Zhu, R.-Y.; He, J.; Wang, X.-C.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 13194.
(f) Chen, K.; Shi, B.-F. Angew. Chem. Int. Ed. 2014, 53, 11950.
[17] (a) Neumann, J. J.; Rakshit, S.; Droge, T.; Glorius, F. Angew. Chem. Int. Ed. 2009, 48, 6892.
(b) He, G.; Zhao, Y.; Zhang, S.; Lu, C.; Chen, G. J. Am. Chem. Soc. 2012, 134, 3.
(c) Nadres, E. T.; Daugulis, O. J. Am. Chem. Soc. 2012, 134, 7.
(d) Ye, X.; He, Z.; Ahmed, T.; Weise, K.; Akhmedov, N. G.; Petersen, J.; Shi, X. Chem. Sci. 2013, 4, 3712.
(e) Wang, C.; Chen, C.; Zhang, J.; Han, J.; Wang, Q.; Guo, K.; Liu, P.; Guan, M.; Yao, Y.; Zhao, Y. Angew. Chem. Int. Ed. 2014, 53, 9884.
[18] (a) He, G.; Zhang, S.-Y.; Nack, W. A.; Li, Q.; Chen, G. Angew. Chem. Int. Ed. 2013, 52, 11124.
(b) Sun, W.-W.; Cao, P.; Mei, R.-Q.; Li, Y.; Ma, Y.-L.; Wu, B. Org. Lett. 2014, 16, 480.
(c) Wang, Z.; Ni, J.; Kuninobu, Y.; Kanai, M. Angew. Chem. Int. Ed. 2014, 53, 3496.
(d) Wu, X.; Zhao, Y.; Zhang, G.; Ge, H. Angew. Chem. Int. Ed. 2014, 53, 3706.
(e) Wu, X.; Zhao, Y.; Ge, H. Chem. Eur. J. 2014, 20, 9530.
(f) Wu, X.; Yang, K.; Zhao, Y.; Sun, H.; Li, G.; Ge, H. Nat. Commun. 2015, 6, 6462.
[19] He, J.; Shigenari, T.; Yu, J.-Q. Angew. Chem. Int. Ed. 2015, 54, 6545.
[20] (a) Rit, R. K.; Yadav, R.; Sahoo, A. K. Org. Lett. 2012, 14, 3724.
(b) Zhou. L.; Lu, W. Org. Lett. 2014, 16, 508.
(c) Li, Q.; Zhang, S.-Y.; He, G.; Nack, W. A.; Chen, G. Adv. Synth. Catal. 2014, 356, 1544.
(d) Wu, X.; Zhao, Y.; Ge, H. Chem. Asian J. 2014, 9, 2736.
(e) Wang, Z.; Kuninobu, Y.; Kanai, M. Org. Lett. 2014, 16, 4790.
(f) Chen, K.; Zhang, S.-Q.; Jiang, H.-Z.; Xu, J.-W.; Shi, B.-F. Chem. Eur. J. 2015, 21, 3264.
[21] (a) Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2012, 134, 7313.
(b) Chen, F.-J.; Zhao, S.; Hu, F.; Chen, K.; Zhang, Q.; Zhang, S.-Q.; Shi, B.-F. Chem. Sci. 2013, 4, 4187.
(c) Shan, G.; Yang, X.; Zong, Y.; Rao, Y. Angew. Chem. Int. Ed. 2013, 52, 13606.
(d) Zong, Y.; Rao, Y. Org. Lett. 2014, 16, 5278.
[22] (a) Wasa, M.; Yu, J.-Q. J. Am. Chem. Soc. 2008, 130, 14058.
(b) Rit, R. K.; Yadav, M. R.; Ghosh, K.; Shankar, M.; Sahoo, A. K. Org. Lett. 2014, 16, 5258.
(c) Zhang, L.-S.; Chen, G.; Wang, X.; Guo, Q.-Y.; Zhang, X.-S.; Pan, F.; Chen, K.; Shi, Z.-J. Angew. Chem. Int. Ed. 2014, 53, 3899.
(d) Lin, C.; Yu, W.; Yao, J.; Wang, B.; Liu, Z.; Zhang, Y. Org. Lett. 2015, 17, 1340.
(e) Yan, S.-Y.; Liu, Y.-J.; Liu, B.; Liu, Y.-H.; Zhang, Z.-Z.; Shi, B.-F. Chem. Commun. 2015, 51, 7341.
(f) Wang, X.; Qiu, R.; Yan, C.; Reddy, V. P.; Zhu, L.; Xu, X.; Yin, S.-F. Org. Lett. 2015, 17, 1970.
(g) Ye, X.; Petersen, J. L.; Shi, X. Chem. Commun. 2015, 51, 7863.
(h) Xiong, H.-Y.; Besset, T.; Cahard, D.; Pannecoucke, X. J. Org. Chem. 2015, 80, 4204.
/
| 〈 |
|
〉 |