

Rh/Ag双金属催化的碳氢键氧化Heck反应研究
收稿日期: 2015-05-10
网络出版日期: 2015-06-15
基金资助
项目受国家自然科学基金(Nos.21372210,21432009)资助.
Rh/Ag Bimetallic Catalyzed C-H Bond Olefination of Benzonitriles
Received date: 2015-05-10
Online published: 2015-06-15
Supported by
Project supported by the National Natural Science Foundation of China(Nos. 21372210, 21432009).
鲁平 , 冯超 , 罗德平 . Rh/Ag双金属催化的碳氢键氧化Heck反应研究[J]. 化学学报, 2015 , 73(12) : 1315 -1319 . DOI: 10.6023/A15050322
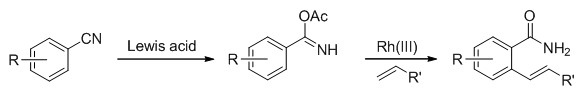
Transition-metal-catalyzed C-H functionalization emerges as an atom-economical and highly efficient strategy in the synthetic organic chemistry. In this regard, oxidative Heck reaction, pioneered by Fujiwara and Moritani, represents a great evolvement to the traditional Heck reaction by obviating the substrate preactivation. While palladium was routinely adopted as the catalyst of choice because of its well established catalytic activities, other transition metals, especially rhodium also proved to be suitable candidate in pursuit of such oxidative C-H alkenylation because of its low catalyst loading, high efficiency and function group tolerance. Under such circumstance, the Rh(Ⅲ)-catalyzed oxidative C-H alkenylation has witness a great advancement in the past decade, with many functionalities such as-COOH,-CONHR, CO2Et, NHAc etc. being explored as suitable directing groups for the initiation of C-H activation. However, most of these directing groups needed prior installation. With our continuing interest in Rh(Ⅲ)-catalyzed C-H functionalization, herein we would like to present a nitrile based bimetallic Rh/Ag catalyzed C-H alkenylation reaction. In this protocol, nitrile was assumed to transform into imine which then took part into the Rh(Ⅲ) catalyzed C-H bond olefination reation. With such reaction cascade, a library of ortho-alkenylated benzamides could be directly obtained from benzonitriles in one pot fashion. All the substrates are commercially available and easy to obtain. A general procedure for the bimetallic Rh/Ag catalyzed C-H olefination of benzonitriles:an oven-dried 10 mL Schlenk tube was charged with nitrile 1(If the nitrile was solid, 0.2 mmol),[RhCp*Cl2]2(6.2 mg, 0.01 mmol), AgSbF6(13.8 mg, 0.04 mmol) in sequence, followed by refilling with N2. Then olefins 2(0.5 mmol), nitriles 1(If the nitrile was liquid, 0.2 mmol) and AcOH were added through syringe. After stirring at 120℃ for 24 h, the reaction mixture was cooled to room temperature and filtered through a celite plug. The solvent was removed in vacuo and the residue was purified by column chromatography to afford the desired product 3.
[1] (a) Moritani, I.; Fujiwara, Y. Tetrahedron Lett. 1967, 8, 1119.
(b) Fujiwara, Y.; Noritani, I.; Danno, S.; Asano, R.; Teranishi, S. J. Am. Chem. Soc. 1969, 91, 7166.
[2] (a) Jia, C.; Lu, W.; Kitamura, T.; Fujiwara, Y. Org. lett. 1999, 1, 2097.
(b) Dams, M.; De Vos, D. E.; Celen, S.; Jacobs, P. A. Angew. Chem. Int. Ed. 2003, 42, 3512.
(c) Tani, M.; Sakaguchi, S.; Ishii, Y. J. Org. Chem. 2004, 69, 1221.
(d) Zhang, Y.-H.; Shi, B.-F.; Yu, J.-Q. J. Am. Chem. Soc. 2009, 131, 5072.
(e) Li, H.; Ding, C.; Xu, B.; Hou, X. Acta Chim. Sinica 2014, 72, 765.(李浩, 丁昌华, 许斌, 侯雪龙, 化学学报, 2014, 72, 765.)
[3] (a) Farrington, E. J.; Brown, J. M.; Barnard, C. F. J.; Rowsell, E. Angew. Chem. Int. Ed. 2002, 41, 169.
(b) Ackermann, L.; Pospech, J. Org. Lett. 2011, 13, 4153.
(c) Ueyama, T.; Mochida, S.; Fukutani, T.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2011, 13, 706.
(d) Kozhushkov, S. I.; Ackermann, L. Chem. Sci. 2013, 4, 886.
[4] (a) Pan, S.; Wakaki, T.; Ryu, N.; Shibata, T. Chem. Asian J. 2014, 9, 1257.
(b) Sevov, C. S.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 10625.
[5] For selected examples of Rh(Ⅲ)-catalyzed C-C bond formation, see:(a) Hesp, K. D.; Bergman, R. G.; Ellman, J. A. J. Am. Chem. Soc. 2011, 133, 11430.
(b) Chan, W.-W.; Lo, S.-F.; Zhou, Z.; Yu, W.-Y. J. Am. Chem. Soc. 2012, 134, 13565.
(c) Gong, T. J.; Xiao, B.; Cheng, W. M.; Su, W.; Xu, J.; Liu, Z. J.; Liu, L.; Fu, Y. J. Am. Chem. Soc. 2013, 135, 10630.
(d) Xie, F.; Qi, Z.; Yu, S.; Li, X. J. Am. Chem. Soc. 2014, 136, 4780.
(e) Song, G.; Li, X. Acc. Chem. Res. 2015, 48, 1007.
[6] For selected examples of Rh(Ⅲ)-catalyzed C-heteroatom bond formation, see:(a) Schroder, N.; Wencel-Delord, J.; Glorius, F. J. Am. Chem. Soc. 2012, 134, 8298.
(b) Wu, K.; Fan, Z.; Xue, Y.; Yao, Q.; Zhang, A. Org. Lett. 2014, 16, 42.
(c) Yang, Y.; Hou, W.; Qin, L.; Du, J.; Feng, H.; Zhou, B.; Li, Y. Chem. Eur. J. 2014, 20, 416.
[7] For selected examples of Rh(Ⅲ)-catalyzed annulation reaction, see:(a) Stuart, D. R.; Bertrand-Laperle, M.; Burgess, K. M.; Fagnou, K. J. Am. Chem. Soc. 2008, 130, 16474.
(b) Tan, X.; Liu, B.; Li, X.; Li, B.; Xu, S.; Song, H.; Wang, B. J. Am. Chem. Soc. 2012, 134, 16163.
(c) Cui, S.; Zhang, Y.; Wang, D.; Wu, Q. Chem. Sci. 2013, 4, 3912.
(d) Shi, X.-Y.; Li, C.-J. Org. Lett. 2013, 15, 1476.
[8] Ueura, K.; Satoh, T.; Miura, M. Org. Lett. 2007, 9, 1407.
[9] (a) Wang, F.; Song, G.; Li, X. Org. Lett. 2010, 12, 5430.
(b) Rakshit, S.; Grohmann, C.; Besset, T.; Glorius, F. J. Am. Chem. Soc. 2011, 133, 2350.
[10] Park, S. H.; Kim, J. Y.; Chang, S. Org. Lett. 2011, 13, 2372.
[11] Patureau, F. W.; Glorius, F. J. Am. Chem. Soc. 2010, 132, 9982.
[12] (a) Choudary, B. M.; Chowdari, N. S.; Madhi, S.; Kantam, M. L. Angew. Chem. Int. Ed. 2001, 40, 4619.
(b) Lee, J. M.; Na, Y.; Han, H.; Chang, S. Chem. Soc. Rev. 2004, 33, 302.
[13] (a) Mitsudome, T.; Mikami, Y.; Mori, H.; Arita, S.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Chem. Commun. 2009, 3258.
(b) Kiss, A.; Hell, Z. Tetrahedron Lett. 2011, 52, 6021.
(c) Li, Z.; Wang, L.; Zhou, X. Adv. Synth. Catal. 2012, 354, 584.
(d) Liu, Y. M.; He, L.; Wang, M. M.; Cao, Y.; He, H. Y.; Fan, K. N. ChemSusChem 2012, 5, 1392.
(e) He, R.; Jin, X.; Chen, H.; Huang, Z. T.; Zheng, Q. Y.; Wang, C. J. Am. Chem. Soc. 2014, 136, 6558. Cyan group can be used as directing group for the C-H bond activation directly, see:
(f) Li, W.; Xu, Z.; Sun, P.; Jiang, X.; Fang, M. Org. Lett. 2011, 13, 1286.
(g) Du, B.; Jiang, X.; Sun, P. J. Org. Chem. 2013, 78, 2786.
(h) Li, W.; Sun, P. J. Org. Chem. 2012, 77, 8362.
[14] Patureau, F. W.; Besset, T.; Glorius, F. Angew. Chem., Int. Ed. 2011, 50, 1064.
[15] Reddy, M. C.; Manikandan, R.; Jeganmohan, M. Chem. Commun. 2013, 49, 6060.
[16] For the spin-spin splitting wasn't clear enough, the exact deuteration rate can't be determined. The NMR information can be found in the Supporting Information.
/
| 〈 |
|
〉 |