

纳米碳纤维的表面改性对水电解析氢反应催化活性的影响
收稿日期: 2015-03-21
网络出版日期: 2015-06-29
基金资助
项目受国家自然科学基金(No. 21073061)和上海市大学生创新训练项目(No. S13028)资助.
Effects of Surface Modification of Carbon Nanofibers on Their Electro- catalytic Activity for Hydrogen Evolution Reaction of Water Electrolysis
Received date: 2015-03-21
Online published: 2015-06-29
Supported by
Supporting information for this article is available free of charge via the Internet at http://sioc-journal.cn.Project supported by the National Natural Science Foundation of China (No. 21073061) and Shanghai university student innovative activities plan.
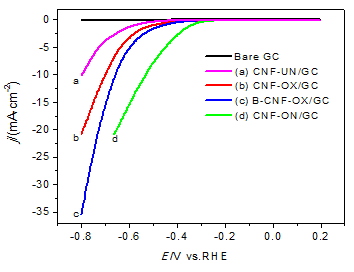
主要研究纳米碳纤维(CNF)的表面改性对析氢反应(HER)催化活性的影响. 首先采用一种简单易行的超声处理方法, 以混酸(浓硫酸和浓硝酸)为溶剂对CNF进行了表面化学处理, 以在其表面引入含氧(CNF-OX)官能团, 然后将CNF-OX在氨水中超声处理, 以引入含氮(CNF-ON)官能团, 以及将CNF-OX和硼酸混合经高温热解对CNF-OX进行掺B处理(B-CNF-OX). XPS结果表明, 超声处理可以成功地在CNF表面引入含氧和含氮官能团, 硼酸高温处理可以掺入B原子. 电化学测试结果表明, 经过表面改性后的CNF的HER催化性能都要好于未处理过的CNF-UN, 其中N掺杂的CNF-ON表现出最好的HER催化活性, 且CNF-OX经掺B处理后, B-CNF-OX的性能也较CNF-OX有所增强, 即三者的催化活性大小如下: CNF-OX
钮东方 , 丁勇 , 马智兴 , 王明辉 , 刘洲 , 张博文 , 张新胜 . 纳米碳纤维的表面改性对水电解析氢反应催化活性的影响[J]. 化学学报, 2015 , 73(7) : 729 -734 . DOI: 10.6023/A15030195
The effects of surface modification of CNF on their electrocatalytic activity for hydrogen evolution reaction (HER) were investigated. Firstly, Oxygen-containing functional groups were introduced onto the CNF surface (labeled as CNF-OX) by a simple sonochemical oxidation in mixed acids (concentrated sulfuric acid and nitric acid) and then the nitrogen-containing functional groups were introduced onto the CNF-OX surface (labeled as CNF-ON) by sonochemical treatment in ammonia, and the B-doped CNF was synthesized by mixing CNF-OX and boric acid in a ratio following by pyrolysis at 800 ℃ under N2 atmosphere (labeled as B-CNF-OX). The XPS results showed that the ultrasonic treatments could introduce oxygen- and nitrogen-containing functional groups onto the CNF surface successfully, the oxygen atoms content of CNF-OX was increased from 1.96% to 6.01% compared to the untreated CNF (CNF-UN), and nitrogen atoms content of CNF-ON was increased from 0% to 1.02% compared to CNF-OX. The pyrolysis of boric acid could introduce B element onto the CNF-OX surface where the B atoms content of B-CNF-OX was 1.00%. The linear sweep voltammetry (LSV) test results showed that the HER electrocatalytic activity of the modified CNFs were higher than CNF-UN, and among them CNF-ON had the highest electrocatalytic activities for HER than the others, the onset overpotential of CNF-ON was 344 mV, which shifted 240 mV positively compared to CNF-UN, and the electrocatalytic activity of B-CNF-OX was enhanced by B-doping treatment compared to CNF-OX. The Tafel test indicated CNF-ON had smallest Tafel slop of 154 mV/dec and largest exchange current density of 6.68 μA/cm2. The electrochemical impedance spectroscopy (EIS) was carried out to investigate the complex interfacial properties of the CNFs modified electrode, and the results showed that CNF-ON had the smallest charge transfer resistance of 1578 Ω among all the catalysts, which means the nitrogen-doped CNF had higher activity than the oxygen- and boron-doped CNF. All above indicated that the doping of heteroatom (O, B, N) onto the CNFs surface had effects on their electrocatalytic activity for HER, especially the introduction of N atom onto the CNF surface had excellent improvement.

[1] Liu, L.; Zha, D.-W.; Wang, Y.; He, J.-B. Int. J. Hydrogen Energy 2014, 39, 14712.
[2] Cao, X.; Han, Y.; Gao, C.; Xu, Y.; Huang, X.; Willander, M.; Wang, N. Nano Energy 2014, 9, 301.
[3] Bessel, C. A.; Laubernds, K.; Rodriguez, N. M.; Baker, R. T. K. J. Phys. Chem. B 2001, 105, 1115.
[4] Aricò, A. S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; Van Schalkwijk, W. Nat. Mater. 2005, 4, 366.
[5] Taylor, R.; Humffray, A. J. Electroanal. Chem. Interfacial Electrochem. 1975, 64, 63.
[6] Zhong, R.-S.; Qin, Y.-H.; Niu, D.-F.; Zhang, X.-S.; Zhou, X.-G.; Sun, S.-G.; Yuan, W.-K. Electrochim. Acta 2013, 89, 157.
[7] Qin, Y.-H.; Jiang, Y.; Niu, D.-F.; Zhang, X.-S.; Zhou, X.-G.; Niu, L.; Yuan, W.-K. J. Power Sources 2012, 215, 130.
[8] Qin, Y.-H.; Li, H.-C.; Yang, H.-H.; Zhang, X.-S.; Zhou, X.-G.; Niu, L.; Yuan, W.-K. J. Power Sources 2011, 196, 159.
[9] Zhang, Z.-G.; Zhao, T.-K.; Li, T.-H.; Wang, J.-B.; Guo, Z.-X. Carbon 2014, 26. (张智广, 赵廷凯, 李铁虎, 王建兵, 郭争光, 炭素, 2014, 26.)
[10] Cao, Y.; Yu, H.; Tan, J.; Peng, F.; Wang, H.; Li, J.; Zheng, W.; Wong, N.-B. Carbon 2013, 57, 433.
[11] Zhao, D.-M.; Li, Z.-W.; Liu, L.-D.; Zhang, Y.-H.; Ren, D.-C.; Li, J. Acta Chim. Sinica 2013, 72, 185. (赵冬梅, 李振伟, 刘领弟, 张艳红, 任德财, 李坚, 化学学报, 2013, 72, 185.)
[12] Lu, Z.-J.; Xu, M.-W.; Bao, S.-J.; Chai, H. Acta Chim. Sinica 2013, 71, 957. (鲁振江, 徐茂文, 包淑娟, 柴卉, 化学学报, 2013, 71, 957.)
[13] Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. ACS nano 2010, 4, 1321.
[14] Li, Y.; Mi, R.; Li, S.; Liu, X.; Ren, W.; Liu, H.; Mei, J.; Lau, W.-M. Int. J. Hydrogen Energy 2014, 39, 16073.
[15] Cheng, Y.; Tian, Y.; Fan, X.; Liu, J.; Yan, C. Electrochim. Acta 2014, 143, 291.
[16] Li, L.-X.; Zhao, H.-W.; Xu, W.-W.; Zhang, Y.-Q.; An, B.-G.; Geng, X. Acta Phys.-Chim. Sin. 2015, 31(3), 498. (李莉香, 赵宏伟, 许微微, 张砚秋, 安百钢, 耿新, 物理化学学报, 2015, 31(3), 498.)
[17] Zhang, J.; Wu, S.; Chen, X.; Pan, M.; Mu, S. J. Power Sources 2014, 271, 522.
[18] Ratso, S.; Kruusenberg, I.; Vikkisk, M.; Joost, U.; Shulga, E.; Kink, I.; Kallio, T.; Tammeveski, K. Carbon 2014, 73, 361.
[19] Zhong, R.-S.; Qin, Y.-H.; Niu, D.-F.; Tian, J.-W.; Zhang, X.-S.; Zhou, X.-G.; Sun, S.-G.; Yuan, W.-K. J. Power Sources 2013, 225, 192.
[20] Moncoffre, N.; Hollinger, G.; Jaffrezic, H.; Marest, G.; Tousset, J. Nucl. Instrum. Methods Phys. Res., Sect. B 1985, 7, 177.
[21] Figueiredo, J. L.; Pereira, M. F. R. Catal. Today 2010, 150, 2.
[22] Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. J. Am. Chem. Soc. 2013, 135, 17881.
[23] Liu, B.; He, J.-B.; Chen, Y.-J.; Wang, Y.; Deng, N. Int. J. Hydrogen Energy 2013, 38, 3130.
[24] Jiang, Y.; Zhang, J.; Qin, Y.-H.; Niu, D.-F.; Zhang, X.-S.; Niu, L.; Zhou, X.-G.; Lu, T.-H.; Yuan, W.-K. J. Power Sources 2011, 196, 9356.
/
| 〈 |
|
〉 |