

高倍率锂离子电池负极材料:氮掺杂碳纳米笼
收稿日期: 2015-04-28
网络出版日期: 2015-07-23
基金资助
项目受国家自然科学基金(Nos.21473089,51232003,21373108,21173115,21203092),“973”项目(No.2013CB932902),苏州市科技计划(No.ZXG2013025)和常州市科技支撑计划(No.CE20130032)资助.
Nitrogen-Doped Carbon Nanocages as High-Rate Anode for Lithium Ion Batteries
Received date: 2015-04-28
Online published: 2015-07-23
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21473089, 51232003, 21373108, 21173115, 21203092), “973” programs (No. 2013CB932902), Suzhou Program (No. ZXG2013025) and Changzhou Technology Support Program (No. CE20130032).
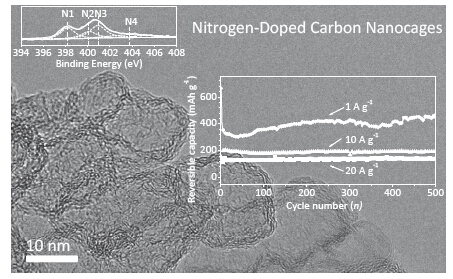
锂离子电池具有能量密度高和循环性好等优点, 广泛应用于小型移动设备等领域, 但尚不能满足需要兼具高容量和高倍率性能的应用要求. 以兼具高比表面积、氮含量高且可调、良好石墨化程度、多尺度分级结构(含孔结构)、有微孔通道的寡层笼壁结构等特征的氮掺杂碳纳米笼(NCNC)为锂离子电池负极材料, 展现出高的比容量、优异的倍率性能和稳定性, 譬如: 在0.1 A·g-1小电流密度下, NCNC800的循环稳定的充电比容量可以高达约900 mAh·g-1, 显著优于商业石墨; 在20.0 A·g-1大电流密度下, 循环500圈后的可逆比容量仍能稳定在约135 mAh·g-1. 如此优异的电化学性能可归因于NCNC的结构特征, 如高比表面积、良好石墨化程度、独特介观结构和孔结构, 这些特征有利于锂离子传输、电解液渗透和电子传导等. 这为开发高倍率和高比容量的锂离子电池负极材料提供思路.
吕之阳 , 冯瑞 , 赵进 , 范豪 , 徐丹 , 吴强 , 杨立军 , 陈强 , 王喜章 , 胡征 . 高倍率锂离子电池负极材料:氮掺杂碳纳米笼[J]. 化学学报, 2015 , 73(10) : 1013 -1017 . DOI: 10.6023/A15040289
The specific power or rate capability of lithium-ion batteries (LIBs) today is insufficient for electric vehicles requiring fast charging and high power output. Compared with great progresses in the high-rate cathodes of LIBs, the development of matchable high-rate anodes still becomes a huge challenge. Carbonaceous materials have been widely used as the anodes of commercial LIBs due to the good conductivity, low cost and environmental friendliness. The nanostructured carbon materials could achieve the high surface area for electrolyte penetration and the short solid-state ion diffusion length for rapid ion transport. Moreover, carbon nanomaterials avoid the large volume change during lithiation/delithiation, which is grave problem for the other high capacity anode materials including Si-based materials and metal oxides. In addition, heteroatom-doping can provide further space for the optimization of lithium storage performance. Herein, we reported the nitrogen-doped carbon nanocages (NCNC) as high-performance anode materials for lithium ion batteries, prepared by an in-situ generated magnesium oxide template method (from decomposition of basic magnesium carbonate) and pyridine as carbon source at growth temperatures of 700, 800, and 900 ℃. The morphology, structure, and electrochemical performances were investigated by transmission electron microscope (TEM), X-ray photoelectron spectroscopy (XPS), nitrogen adsorption/desorption equipment, and constant current discharge/charge tests. For example, NCNC800, prepared at 800 ℃, has a high specific surface area (1197 m2·g-1), high nitrogen content (8.2 at%), good conductivity (46 S·m-1) and mesopores structure. As an anode material for LIBs, NCNC800 delivers a steady charge capacity of ca. 900 mAh·g-1 at a low current density of 0.1 A·g-1 after 20 cycles activation, and stabilizes at ca. 135 mAh·g-1 even over 500 cycles at a high rate of 20.0 A·g-1, which are superior to graphene and graphite. These good electrochemical performances, such as high steady charge capacity, high-rate capability and excellent cyclability, can be ascribed to the unique structure of NCNC, which is beneficial to the electrolyte penetration, Li-ion diffusion, electron conduction, and structural stability during lithium ions intercalation/deintercalation. This unique carbon nanomaterial provides a new alternative of anode materials for high-performance LIBs.

[1] Armand, M.; Tarascon, J. M. Nature 2008, 451, 652.
[2] Xu, Y.; Guo, J.; Wang, C. J. Mater. Chem. 2012, 22, 9562.
[3] Goodenough, J. B.; Park, K. S. J. Am. Chem. Soc. 2013, 135, 1167.
[4] Shen, L. F.; Uchaker, E.; Zhang, X. G.; Cao, G. Z. Adv. Mater. 2012, 24, 6502.
[5] Kang, B.; Ceder, G. Nature 2009, 458, 190.
[6] Wu, X. L.; Jiang, L. Y.; Cao, F. F.; Guo, Y. G.; Wan, L. J. Adv. Mater. 2009, 21, 2710.
[7] Wang, L.; He X, Sun W.; Wang, J.; Li, Y.; Fan, S. Nano Lett. 2012, 12, 5632.
[8] Ye, Y.; Zhu, J. Y.; Yao, Y. N.; Wang, Y. G.; Wu, P.; Tang, Y. W.; Zhou, Y. M.; Lu, T. H. Acta Chim. Sinica 2015, 73, 151. (叶亚, 朱婧怡, 姚依男, 王雨果, 吴平, 唐亚文, 周益明, 陆天虹, 化学学报, 2015, 73, 151.)
[9] Manthiram, A. J. Phys. Chem. Lett. 2011, 2, 176.
[10] Li, H.; Wang, Z. X.; Chen, L. Q.; Huang, X. J. Adv. Mater. 2009, 21, 4593.
[11] Zhu, G. N.; Liu, H. J.; Zhuang, J. H.; Wang, C. X.; Wang, Y. G.; Xia, Y. Y. Energy Environ. Sci. 2011, 4, 4016.
[12] Zhang, Y.; Hu, X.; Xu, Y.; Ding, M. Acta Chim. Sinica 2013, 71, 1341. (张永龙, 胡学步, 徐云兰, 丁明亮, 化学学报, 2013, 71, 1341.)
[13] Zhang, F. Ph.D. Dissertation, Jilin University, Jilin, 2009 (张峰, 博士论文, 吉林大学, 吉林, 2009).
[14] Ng, S. H.; Wang, J.; Guo, Z. P.; Chen, J.; Wang, G. X.; Liu, H. K. Electrochim. Acta 2005, 51, 23.
[15] Li, C. C.; Yin, X. M.; Chen, L. B.; Li, Q. H.; Wang, T. L. J. Phys. Chem. C 2009, 113, 13438.
[16] Han, F. D.; Yao, B.; Bai, Y. J. J. Phys. Chem. C 2011, 115, 8923.
[17] Wu, Z. S.; Ren, W.; Xu, L.; Xu, L.; Li, F.; Cheng, H. M. ACS Nano 2011, 5, 5463.
[18] Fang, Y.; Lv, Y.; Che, R.; Wu, H.; Zhang, X.; Gu, D.; Zheng, G.; Zhao, D. J. Am. Chem. Soc. 2013, 135, 1524.
[19] Yang, L. J.; Jiang, S. J.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X. Z.; Wu, Q.; Ma, J.; Ma, Y. W.; Hu, Z. Angew. Chem., Int. Ed. 2011, 50, 7132.
[20] Zhao, Y.; Yang, L. J.; Chen, S.; Wang, X. Z.; Ma, Y. W.; Wu, Q.; Jiang, Y.; Qian, W. J.; Hu, Z. J. Am. Chem. Soc. 2013, 135, 1201.
[21] Ma, Y. W.; Sun, L. Y.; Huang, W.; Zhang, L. R.; Zhao, J.; Fan, Q. L.; Huang, W. J. Phys. Chem. C 2011, 115, 24592.
[22] Long, Q.; Chen, W. M.; Wang, Z. H.; Shao, Q. G.; Li, X.; Yuan, L. X.; Hu, X. L.; Zhang, W. X.; Huang, Y. H. Adv. Mater. 2012, 24, 2047.
[23] Shin, W. H.; Jeong, H. M.; Kim, B. G.; Kang, J. K.; Choi, J. W. Nano Lett. 2012, 12, 2283.
[24] Xie, K.; Qin, X. T.; Wang, X. Z.; Wang, Y. N.; Tao, H. S.; Wu, Q.; Yang, L. J.; Hu, Z. Adv. Mater. 2012, 24, 347.
[25] Chen, S.; Bi, J. Y.; Zhao, Y.; Yang, L. J.; Zhang, C.; Ma, Y. W.; Wu, Q.; Wang, X. Z.; Hu, Z. Adv. Mater. 2012, 24, 5593.
[26] Feng, R.; Wang, L. W.; Lyu, Z. Y.; Wu, Q.; Yang, L. J.; Wang, X. Z.; Hu, Z. Acta Chim. Sinica 2014, 72, 653. (冯瑞, 王立伟, 吕之阳, 吴强, 杨立军, 王喜章, 胡征, 化学学报, 2014, 72, 653.)
[27] Kaskhedikar, N. A.; Maier, J. Adv. Mater. 2009, 21, 2664.
[28] Wen, L.; Liu, C. M.; Song, R. S.; Luo, H. Z.; Shi, Y.; Li, F.; Chen, H. M. Acta Chim. Sinica 2014, 72, 333. (闻雷, 刘成名, 宋仁升, 罗洪泽, 石颖, 李峰, 成会明, 化学学报, 2014, 72, 333.)
[29] Liu, N.; Hu, L.; McDowell, M. T.; Jackson, A.; Cui, Y. ACS Nano 2011, 5, 657.
[30] Hou, J. H.; Cao, C. B.; Idrees, F.; Ma, X. L. ACS Nano 2015, 9, 2556.
[31] Song, H. W.; Yang, G. Z.; Wang, C. X. ACS Appl. Mater. Interfaces 2014, 6, 21661.
[32] Wu, Z. S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J. P.; Chen, Z. P.; Zhou, G. M.; Li, F.; Cheng, H. M. ACS Nano 2010, 4, 3187.
[33] Lyu, Z. Y.; Xu, D.; Yang, L. J.; Che, R. C.; Feng, R.; Zhao, J.; Li, Y.; Wu, Q.; Wang, X. Z.; Hu, Z. Nano Energy 2015, 12, 657.
/
| 〈 |
|
〉 |