

太赫兹光谱和密度泛函理论(DFT)分析呋喃妥因和尿素共晶体
收稿日期: 2015-05-22
网络出版日期: 2015-08-18
基金资助
项目受国家自然科学基金(No.21205110)和浙江省自然科学基金(No.LY15B050004)资助
Co-crystal between Nitrofurantion and Urea Investigated by Terahertz Spectroscopy and Density Functional Theory
Received date: 2015-05-22
Online published: 2015-08-18
Supported by
Project supported by the National Natural Science Foundation of China (No. 21205110) and Natural Science Foundation of Zhejiang Province (No. LY15B050004)
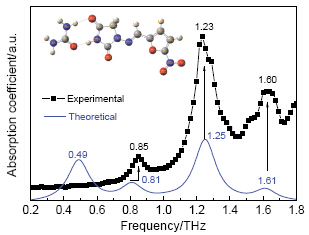
利用太赫兹时域光谱(Terahertz time-domain spectroscopy, THz-TDS)技术对呋喃妥因、尿素及其研磨和溶剂共晶体进行表征分析, 实验结果显示了呋喃妥因和尿素的研磨和溶剂共晶体位于0.85、1.23、1.60 THz的吸收峰明显区别于原料物质. 该结果表明太赫兹光谱技术可以有效鉴别呋喃妥因、尿素及其共晶体. 运用密度泛函理论(Density functional theory, DFT)对呋喃妥因和尿素共晶体的2种可能结构进行了结构优化和光谱模拟, 模拟结果显示其中的结构A在0.49、0.81、1.25、1.61 THz处具有吸收峰, 与实验结果较吻合. 推断共晶体氢键的形成位置为尿素中的氨基H6和呋喃妥因上的酰胺基O30, 该处形成第一处氢键, 而呋喃妥因的酰胺基H31和尿素上的羰基O1形成第二处氢键. 同时结合理论模拟结果对呋喃妥因和尿素共晶体分子振动模式进行归属.
张琪 , 方虹霞 , 张慧丽 , 秦丹 , 洪治 , 杜勇 . 太赫兹光谱和密度泛函理论(DFT)分析呋喃妥因和尿素共晶体[J]. 化学学报, 2015 , 73(10) : 1069 -1073 . DOI: 10.6023/A15050351
Terahertz time-domain spectroscopy (THz-TDS) technology was utilized for the characterization and analysis of nitrofurantion, urea, and their solvent/grinding co-crystals. Experimental results showed that similar absorption peaks of its solvent and grinding co-crystals which formed by nitrofurantion and urea at 0.85, 1.23 and 1.60 THz can be observed, indicating that they are same materials. The position and intensity of absorption peaks for such co-crystals are significantly different from corresponding original materials. The result demonstrated that THz-TDS technique could effectively identify and characterize nitrofurantion, urea and its co-crystal. The optimized geometries and vibrational spectra of the two feasible theoretical molecular structures of nitrofurantion-urea co-crystal were performed with the density functional theory (DFT) calculation. Geometry optimization was obtained by choosing B3LYP density functional with the atom-centered Gaussian-type 6-31G (d, p) basis set. The theoretical result showed that absorption peaks at 0.49, 0.81, 1.25, 1.61 THz occurred in structure A, which is pretty agreement with the THz experimental result. Comparing the experimental and theoretical results, it is confirmed that the first hydrogen bond is formed at place between amino H6 of urea and amide O30 of nitrofurantion, while the second one is constituted by amide H31 of nitrofurantion and carbonyl O1 of urea. The vibrational modes of nitrofurantion-urea co-crystal were also assigned with the help of the simulated DFT results. The different vibrational mode assignment for nitrofurantion-urea co-crystal indicates that the main vibrational features of such co-crystal lie on the ring and group out-of-plane bending vibrations in THz region. This work provides experimental and theoretical benchmark to detect and analyze the hydrogen bonding interaction and molecular structure of co-crystals in the pharmaceutical field with the newly developing terahertz spectroscopic technology.

Key words: nitrofurantion; urea; co-crystal; hydrogen bond; Terahertz time-domain spectroscopy
[1] Tous, S. S.; Mohammed, F. A.; Sayed, M. A. J. Drug Delivery Sci. Technol. 2008, 18, 367.
[2] Li, W.; Chen, J.; Xiang, B. R.; An, D. K. Acta Chim. Sinica 2001, 59, 109. (李伟, 陈坚, 相秉仁, 安登魁, 化学学报, 2001, 59, 109.)
[3] Eyjolfsson, R. Drug Dev. Ind. Pharm. 1999, 25, 105.
[4] Otsuka, M.; Matsuda, Y. J. Pharm. Pharmacol. 1993, 45, 406.
[5] Schultheiss, N.; Newman, A. Cryst. Growth Des. 2009, 9, 2950.
[6] Desiraju, G. R. J. Am. Chem. Soc. 2013, 135, 9952.
[7] Vishweshwar, P.; McMahon, J. A.; Bis, J. A.; Zaworotko, M. J. J. Pharm. Sci. 2006, 95, 499.
[8] Du, Y.; Xia, Y.; Zhang, H.; Hong, Z. Spectrochim. Acta, Part A 2013, 111, 192.
[9] Agbolaghi, S.; Abbasi, F.; Abbaspoor, S.; Alizadeh-Osgouei, M. Eur. Polym. J. 2015, 66, 108.
[10] Alhalaweh, A.; Roy, L.; Rodriguez-Hornedo, N.; Velaga, S. P. Mol. Pharm. 2012, 9, 2605.
[11] Vangala, V. R.; Chow, P. S.; Tan, R. B. H. Cryst. Growth Des. 2012, 12, 5925.
[12] Alhalaweh, A.; George, S.; Basavoju, S.; Childs, S. L.; Rizvi, S. A. A.; Velaga, S. P. Cryst. Eng. Commun. 2012, 14, 5078.
[13] Tutughamiarso, M.; Bolte, M.; Wagner, G.; Egert, E. Acta Crystallogr. Sect. C: Cryst. Struct. Commun. 2011, 67, O18.
[14] Vangala, V. R.; Chow, P. S.; Tan, R. B. H. Cryst. Eng. Commun. 2011, 13, 759.
[15] Cherukuvada, S.; Babu, N. J.; Nangia, A. J. Pharm. Sci. 2011, 100, 3233.
[16] Aaltonen, J.; Strachan, C. J.; Pollanen, K.; Yliruusi, J.; Rantanen, J. J. Pharm. Biomed. Anal. 2007, 44, 477.
[17] Li, X. R.; Guo, W.; Lu, Y.; Acta Chim. Sinica 2008, 66, 515. (李向荣, 郭伟, 卢雁, 化学学报, 2008, 66, 515.)
[18] Jin, T. S.; Zhao, Y.; Liu, L. B.; Li, T. S. Chin. J. Org. Chem. 2006, 26, 975. (靳通收, 赵莹, 刘利宾, 李同双, 有机化学, 2006, 26, 975.)
[19] Yang, J.; Li, S.; Zhao, H.; Song, B.; Zhang, G.; Zhang, J.; Zhu, Y.; Han, J. J. Phys. Chem. A 2014, 118, 10927.
[20] Xu, J. Z.; Zhang, X. C. Technology and Applications of Terahertz Wave, Peking University Press, Beijing, 2007, pp. 1~5. (许景周, 张希成, 太赫兹波科学技术与应用, 北京大学出版社, 北京, 2007, pp. 1~5.)
[21] Delaney, S. P.; Korter, T. M. J. Phys. Chem. A 2015, 119, 3269.
[22] King, M. D.; Korter, T. M. Cryst. Growth Des. 2011, 11, 2006.
[23] Du, Y.; Zhang, H.; Xue, J.; Fang, H.; Zhang, Q.; Xia, Y.; Li, Y.; Hong, Z. Spectrochim. Acta, Part A 2015, 139, 488.
[24] Kang, X. S.; Hou, D. B.; Zhang, G. X.; Chen, X. A.; Yue, F. H.; Huang, P. J.; Zhou, Z. K. Spectrosc. Spec. Anal. 2012, 32, 1744. (康旭升, 侯迪波, 张光新, 陈锡爱, 岳飞亨, 黄平捷, 周泽魁, 光谱学与光谱分析, 2012, 32, 1744.)
[25] King, M. D.; Davis, E. A.; Smith, T. M.; Korter, T. M. J. Phys. Chem. A. 2011, 115, 11039.
[26] Bertolasi, V.; Gilli, P.; Ferretti, V.; Gilli, G. Acta Crystallogr. Sect. C: Cryst. Struct. Commun. 1993, 49, 741.
[27] Fang, H. X.; Zhang, Q.; Zhang, H. L.; Du, Y.; Hong, Z. Acta Phys.-Chim. Sin. 2015, 31, 22. (方虹霞, 张琪, 张慧丽, 杜勇, 洪治, 物理化学学报, 2015, 31, 22.)
[28] Wang, X. M.; Wang, W. N. Acta Chim. Sinica 2008, 66, 2248. (王雪美, 王卫宁, 化学学报, 2008, 66, 2248.)
[29] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, Jr. J. A.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Bakken, V.; Damo, C.; Jaramillo, J.; Gomperts, R.; Tratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 03, Revision E. 01; Gaussian, Inc., Wallingford, CT, 2004.
[30] Islam, S. M.; Huelin, S. D.; Dawe, M.; Poirier, R. A. J. Chem. Theory Comput. 2008, 4, 86.
/
| 〈 |
|
〉 |