

二维Bi2Se3晶体对罗丹明6G分子的荧光猝灭效应研究
收稿日期: 2015-07-28
网络出版日期: 2015-09-15
基金资助
项目受国家重大科学研究计划(Nos. 2014CB932500, 2011CB921904, 2013CB932603)、国家自然科学基金(Nos. 21173004, 21222303, 51121091)和中组部“万人计划”青年拔尖人才计划资助.
Fluorescence Quenching Effect of Rhodanmine 6G on Two-Dimensional Bi2Se3 Crystals
Received date: 2015-07-28
Online published: 2015-09-15
Supported by
Project supported by the National Basic Research Program of China (Nos. 2014CB932500, 2011CB921904, and 2013CB932603), the National Natural Science Foundation of China (Nos. 21173004, 21222303, and 51121091), and National Program for Support of Top-Notch Young Professionals.
吴金雄 , 刘忠范 , 彭海琳 . 二维Bi2Se3晶体对罗丹明6G分子的荧光猝灭效应研究[J]. 化学学报, 2015 , 73(9) : 944 -948 . DOI: 10.6023/A15070513
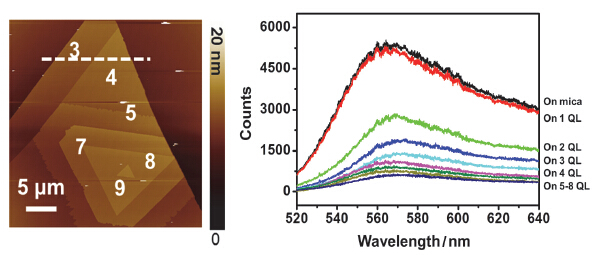
As a novel quantum material, two-dimensional crystal of Bi2Se3 is a typical topological insulator with an insulating bulk gap and gapless edges or surface states, which has created new opportunities for spintronics and low-dissipation electric transport. For the first time, we studied the fluorescence quenching effect of Rhodanmine 6G molecules on two-dimensional crystals of Bi2Se3 with the thickness ranged from 1 layer to 8 layers. Atomically-thin, high-quality two-dimensional crystals of Bi2Se3 with different thicknesses down to monolayer were firstly grown on mica substrates via van der Waals epitaxy. Typically, its domain size is about tens or several tens micrometers, providing a good platform for studying the thickness-dependent chemical and physical properties of two-dimensional Bi2Se3 crystals. Besides, as fluorescence-probe molecules with high fluorescence quantum yield, a uniform and continuous film of Rhodamine 6G molecules were deposited onto the surface of mica substrate and two-dimensional Bi2Se3 crystals by vacuum evaporation, on which adsorption of almost equal number of Rhodamine 6G molecules was achieved revealed by atomic force microscopy characterization. Subsequently, the measurements of fluorescence spectroscopy were conducted on the Horiba-Jobin Yvon Labram HR800 micro Raman spectrometer with a spatial resolution about 1 μm. Excited by 514.5 nm Ar+ laser, the fluorescence signal from 520 nm to 640 nm of Rhodanmine 6G molecules on different surfaces was collected under the same condition. It was found that the atomically-thin Bi2Se3 crystals can quench the fluorescence of adsorbed Rhodamine 6G molecules. Meanwhile, the thicker Bi2Se3 crystals show more obvious quenching ability to Rhodanmine 6G molecules. In the end, a possible quenching mechanism of fluorescence resonance energy transfer was suggested. According to the UV-Vis spectroscopy of two-dimensional crystals of Bi2Se3, the absorption spectroscopy of Bi2Se3 showed a large spectral overlap with the emission fluorescent of Rhodanmine 6G, which is the key to fluorescence resonance energy transfer.
[1] Castro Neto, A. H.; Guinea, F.; Peres, N. M. R.; Novoselov, K. S.; Geim, A. K. Rev. Mod. Phys. 2009, 81, 109.
[2] Liu, Z. F. Acta Chim. Sinica 2014, 72, 267. (刘忠范, 化学学报, 2014, 72, 267.)
[3] Qi, X. L.; Zhang, S. C. Rev. Mod. Phys. 2011, 83, DOI: 10.1103/ RevModPhys.83.1057.
[4] Xie, L. M.; Ling, X.; Fang, Y.; Zhang, J.; Liu, Z. F. J. Am. Chem. Soc. 2009, 131, 9890.
[5] Kim, J.; Cote, L. J.; Kim, F.; Huang, J. J. Am. Chem. Soc. 2010, 132, 260.
[6] Chen, Z. Y.; Berciaud, S.; Nuckolls, C.; Heinz, T. F.; Brus, L. E. ACS Nano 2010, 4, 2964.
[7] Ghaemi, P.; Mong, R. S. K.; Moore, J. E. Phys. Rev. Lett. 2010, 105, 166603.
[8] Sun, Y.; Cheng, H.; Gao, S.; Liu, Q.; Sun, Z.; Xiao, C.; Wu, C.; Wei, S.; Xie, Y. J. Am. Chem. Soc. 2012, 134, 20294.
[9] Xia, Y.; Qian, D.; Hsieh, D.; Wray, L.; Pal, A.; Lin, H.; Bansil, A.; Grauer, D.; Hor, Y. S.; Cava, R. J.; Hasan, M. Z. Nat. Phys. 2009, 5, 398.
[10] Zhang, H. J.; Liu, C. X.; Qi, X. L.; Dai, X.; Fang, Z.; Zhang, S. C. Nat. Phys. 2009, 5, 438.
[11] Zhang, G. H.; Qin, H. J.; Teng, J.; Guo, J. D.; Guo, Q. L.; Dai, X.; Fang, Z.; Wu, K. H. Appl. Phys. Lett. 2009, 95, 053114.
[12] Peng, H. L.; Dang, W. H.; Cao, J.; Chen, Y. L.; Wu, W.; Zheng, W. S.; Li, H.; Shen, Z. X.; Liu, Z. F. Nat. Chem. 2012, 4, 281.
[13] Li, H.; Cao, J.; Zheng, W. S.; Chen, Y. L.; Wu, D.; Dang, W. H.; Wang, K.; Peng, H. L.; Liu, Z. F. J. Am. Chem. Soc. 2012, 134, 6132.
[14] Zhao, S.; Wang, H.; Zhou, Y.; Liao, L.; Jiang, Y.; Yang, X.; Chen, G.; Lin, M.; Wang, Y.; Peng, H.; Liu, Z. Nano Res. 2015, 8, 288.
[15] Dong, H. F.; Gao, W. C.; Yan, F.; Ji, H. X.; Ju, H. X. Anal. Chem. 2010, 82, 5511.
[16] Swathi, R. S.; Sebastian, K. L. J. Chem. Phys. 2008, 129, 054703.
[17] Wang, Y. L.; Lu, J. P.; Tong, Z. F.; Chen, L. Acta Chim. Sinica 2009, 67, 2222. (王益林, 陆建平, 童张法, 陈璐, 化学学报, 2009, 67, 2222.)
[18] Wu, P. G.; Brand, L. Anal. Biochem. 1994, 218, 1.
[19] Clegg, R. M. Curr. Opin. Biotechnol. 1995, 6, 103.
[20] Selvin, P. R. Biochem. Spectrosc. 1995, 246, 300.
[21] Yao, J.; Koski, K. J.; Luo, W. D.; Cha, J. J.; Hu, L. B.; Kong, D. S.; Narasimhan, V. K.; Huo, K. F.; Cui, Y. Nat. Commun. 2014, 5, 5670.
/
| 〈 |
|
〉 |