

一种弱阳离子交换材料的亲水模式糖肽富集新方法
收稿日期: 2015-05-29
网络出版日期: 2015-11-19
基金资助
项目受国家自然科学基金(Nos.81171486,21475129)、辽宁省特聘教授资助.
A Novel Glycopeptides Enrichment Method Using Weak Cation Exchange Chromatography Material under Hydrophilic Interaction Liquid Chromatography Mode
Received date: 2015-05-29
Online published: 2015-11-19
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 81171486, 21475129) and Distinguished Professor of Liaoning Province.
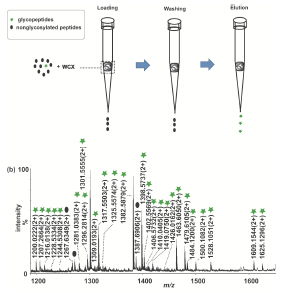
亲水作用色谱(HILIC)材料在糖肽的富集与分离中得到越来越广泛的应用. 针对目前HILIC材料糖肽选择性不足的缺点, 发展新的糖肽富集方法十分必要. 本工作发展了一种商品化的弱阳离子交换材料(WCX)在亲水模式下对糖肽的富集方法. 首先考察了微固相萃取(SPE)模式下WCX上多肽保留的机理. 结果显示, WCX上糖肽的保留同时受乙腈含量和pH的影响. 将WCX用于牛胎球蛋白(fetuin)胰蛋白酶酶解液中糖肽的富集, 获得了39条糖肽信号. 当fetuin与牛血清白蛋白(BSA)酶解液以物质的量比1:5混合时, 富集到26条糖肽信号. 富集人免疫球蛋白(IgG)胰蛋白酶酶解液时获得25条糖肽信号, 1:5时获得23条糖肽信号. 实验说明WCX对唾液酸化和非唾液酸化糖肽的富集均有高选择性. 该方法拓展了WCX的应用范围, 丰富了HILIC材料的种类.
李先琴 , 俞冬萍 , 丰小敏 , 郭志谋 , 李秀玲 , 邹丽娟 , 梁鑫淼 . 一种弱阳离子交换材料的亲水模式糖肽富集新方法[J]. 化学学报, 2015 , 73(10) : 1074 -1079 . DOI: 10.6023/A15050370
Protein glycosylation is a vital bio-process in organisms. Selective enrichment of glycopeptides before mass spectrometry (MS) plays an important role in proteomics owing to the low glycopeptides proportion and the suppression effect of the non-glycosylated peptides. There are several methods used for the enrichment of glycopeptides with their own merits and drawbacks. Among them, hydrophilic interaction liquid chromatography (HILIC) is increasingly applied to glycopeptides enrichment with its promising prospects. In this paper, a new strategy for the enrichment of glycopeptides using a commercial weak cation exchange chromatography (WCX) material under HILIC mode was developed. The influence of concentration of acetonitrile (ACN) and pH value on the retention of tryptic fetuin digests on WCX were studied. Results indicated that non-glycosylated peptides on WCX micro-column were eluted prior to glycopeptides when the concentration of ACN gradually decreased. Furthermore, the hydropathy index of peptides contained in glycopeptides also affected the retention. The bigger the index was, the later the plycopeptides eluted. Meanwhile, the glycopeptides were retained more strongly at lower pH than higher pH. When using WCX to enrich glycopeptides, it was found that not only sialic acid containing glycopeptides but also neutral ones could be selectively enriched. In the enrichment of tryptic IgG digests, 25 glycopeptides were found by WCX while only 1 glycopeptides were found before enrichment. And when the tryptic fetuin digests were enriched by WCX, the glycopeptides numbers detected by MS increased to 39, in contrast to only 2 before enrichment. What's more, WCX can enrich glycopeptides even under relatively complex surrounding. 23 IgG glycopeptides and 26 fetuin glycopeptides could be enriched by WCX when they were respectively mixed with bovine serum albumin (BSA) at a molar ratio of 1:5. The results indicated that glycopeptides could be selectively enriched by WCX under HILIC mode. This is a new glycopeptides enrichment method, which expands the application of WCX and enriches the variety of HILIC material.

[1] Apweiler, R.; Hermjakob, H.; Sharon, N. Biochim. Biophys. Acta 1999, 1473, 4.
[2] Kazuaki, O.; Marth, J. D. Cell 2006, 19, 855.
[3] Chandler, K.; Goldman, R. Mol. Cell. Proteomics 2013, 12(4), 836.
[4] Pinho, S. S.; Carvalho, S.; Marcos-Pinto, R.; Magalhaes, A.; Oliveira, C.; Gu, J.; Dinis-Ribeiro, M.; Carneiro, F.; Seruca, R.; Reis, C. A. Trends Mol. Med. 2013, 19(11), 664.
[5] Padler-Karavani, V. Cancer Lett. 2014, 352, 102.
[6] Kawakami, S.; Hashida, M. J. Controlled Release 2014, 190, 542.
[7] Yu, L.; Li, X. L.; Guo, Z. M.; Zhang, X. L.; Liang, X. M. Chem. Eur. J. 2009, 15, 46.
[8] Calvano, C. D.; Zamboninb, C. G.; Jensen, O. N. J. Proteomics 2008, 71, 304.
[9] Kullolli, M.; Hancock, W. S.; Hincapie, M. Anal. Chem. 2010, 82, 115.
[10] Shi, Z. M.; Fan, C.; Huang, J. J.; Bai, H. H.; Qin, W. J.; Cai, Y.; Qian, X. H. Chin. J. Chromatogr. 2015, 33(2), 116 (时照梅, 范超, 黄俊杰, 白海红, 秦伟捷, 蔡耘, 钱小红, 色谱, 2015, 33, 116.)
[11] Liu, L. T.; Zhang, Y.; Jiao, J.; Yang, P. Y; Lu, H. J. Acta Chim. Sinica 2013, 71, 535. (刘丽婷, 张莹, 焦竞, 杨芃原, 陆豪杰, 化学学报, 2013, 71, 535.)
[12] Liu, L. T.; Zhang, Y.; Zhang, L.; Yan, G. Q.; Yao, J.; Yang, P. Y.; Lu, H. J. Anal. Chim. Acta 2012, 753, 64.
[13] Wang, Y. L.; Liu, M. B.; Xie, L. Q.; Fang, C. Y.; Xiong, H. M.; Lu, H. J. Anal. Chem. 2014, 86, 2057.
[14] Zhao, X.; Jiang, W. H ; Yu, L.; Zou, L. J.; Li, X. L.; Liang, X. M. Acta Chim. Sinica 2013, 71, 343. (赵旭, 姜武辉, 于龙, 邹丽娟, 李秀玲, 梁鑫淼, 化学学报, 2013, 71, 343.)
[15] Li, X. L.; Shen, G. B.; Zhang, F. F.; Yang, B. C; Liang, X. M. J. Chromatogr. B 2013, 941, 45.
[16] Zhang, Y.; Go, E. P.; Desaire, H. Anal. Chem. 2008, 80, 3144.
[17] Takegawa, Y.; Deguchi, K.; Ito, H.; Keira, T.; Nakagawa, H.; Nishimura, S. I. J. Sep. Sci. 2006, 29, 2533.
[18] Liang, X. M. Chin. J. Chromatogr. 2011, 29(3), 191 (梁鑫淼, 色谱, 2011, 29(3), 191.)
[19] Buszewski, B.; Noga, S. Anal. Bioanal. Chem. 2012, 402, 23.
[20] Selman, M. H. J.; McDonnell, L. A.; Palmblad, M.; Renee Ruhaak, L.; Deelder, A. M.; Wuhrer, M. Anal. Chem. 2010, 82, 1073.
/
| 〈 |
|
〉 |