

可见光催化的高炔丙醇经过1,4芳基迁移实现二氟烷基化反应
收稿日期: 2016-07-30
修回日期: 2016-09-13
网络出版日期: 2016-09-18
基金资助
项目受国家自然科学基金(Nos.21172106,21174061,21474048,21372114)资助.
Visible Light Promoted Carbodifluoroalkylation of Homopropargylic Alcohols via Concomitant 1,4-Aryl Migration
Received date: 2016-07-30
Revised date: 2016-09-13
Online published: 2016-09-18
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172106, 21174061, 21474048 and 21372114).
周能能 , 胥攀 , 李伟鹏 , 成义祥 , 朱成建 . 可见光催化的高炔丙醇经过1,4芳基迁移实现二氟烷基化反应[J]. 化学学报, 2017 , 75(1) : 60 -65 . DOI: 10.6023/A16070375
Fluorinated compounds have gained much attention because of their unique electronegativity, metabolic stability and bioavailability, and thus, the synthesis of organofluorine compounds has found wide applications in pharmaceuticals, agrochemicals, and materials science. Among them, the incorporation of a difluoromethyl group (CF2) into organic compounds is of great concern in medicinal chemistry owing to its isosterism with the hydroxyl group. Therefore, the development of new difluoroalkylation methods has attracted great interest in synthetic organic chemistry. Visible light-driven photocatalysis as an eco-friendly and powerful theme has been widely utilized in organic synthesis. In particular, free radical fluorination is emerging as a powerful tool for C-F bond formation, especially under the catalysis of visible light. Recent progress on the visible light-promoted directing difluoroalkylation using ethyl bromodifluoroacetate provided an efficient approach to the target. Herein, we report a contribution towards visible light induced carbodifluoroalkylation of homopropargylic alcohols with the use of ethyl bromodifluoroacetate as a source of difluorinated moieties. This strategy provides a facile way to access functional-difluorinated alkenes through a tandem radical difluoroalkylation and 1,4-aryl migration process. A representative procedure for this reaction is as following:An oven-dried Schlenk tube (10 mL) was equipped with a magnetic stir bar, homopropargylic alcohols (0.2 mmol), fac-Ir(ppy)3 (0.02 equiv., 0.004 mmol), 2-bromo-2,2-difluoroacetate (2.5 equiv. 0.5 mmol), Na2HPO4 (2 equiv., 0.4 mmol). The flask was evacuated and backfilled with Ar for 3 times. 0.5 mL of dry DMA and 0.5 mL of dry DCE were added with syringe under Ar. The tube was placed at a distance (app. 5 cm) from 33 W fluorescent light bulb, and the resulting solution was stirred at ambient temperature under visible-light irradiation. After the reaction was finished, the mixture was then diluted with MTBE (20 mL×2) and water. The combined organic layers were dried over sodium sulfate and the solvent concentrated in vacuo and the residue was purified by chromatography on silica gel to afford the corresponding products.
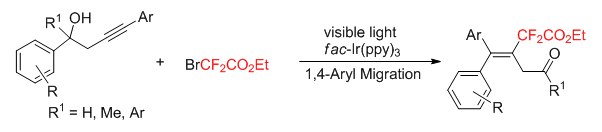
[1] (a) Filler, R.; Kobayashi, Y.; Yagupolskii, L. M. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications, Elsevier, Amsterdam, 1993.
(b) Romanenko, V. D.; Kukhar, V. P. Chem. Rev. 2006, 106, 3868.
(c) Muller, K.; Faeh, C.; Diederich, F.; Science 2007, 317, 1881.
(d) Furuya, T.; Kuttruff, C.; Ritter, T.; Curr. Opin. Drug Discovery Dev. 2008, 11, 80.
(e) Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, 2009.
(f) Liang, T.; Neumann, C. N.; Ritter, T. Angew. Chem., Int. Ed. 2013, 52, 8214.
(g) Ni, C.; Zhu, L.; Hu, J. Acta Chim. Sinica 2015, 73, 90. (倪传法, 朱林桂, 胡金波, 化学学报, 2015, 73, 90.).
(h) Zhang, K.; Xu, X.; Qing, F. Chin. J. Org. Chem. 2015, 35, 556. (张柯, 徐修华, 卿凤翎, 有机化学, 2015, 35, 556.)
[2] For some reviews on fluorination, see:(a) Grushin, V. V. Acc. Chem. Res. 2010, 43, 160.
(b) Cahard, D.; Xu, X.; Couve-Bonnaire, S.; Pannecoucke, X. Chem. Soc. Rev. 2010, 39, 558.
(c) Furuya, T.; Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470.
(d) Lin, A.; Huehls, B.; Yang. J. Org. Chem. Front. 2014, 1, 434. For recent examples, see:
(e) Li, W.; Zhu, Y.; Duan, Y.; Zhang, M.; Zhu, C. Adv. Synth. Catal. 2015, 357, 1277.
(f) Shen, X.; Miao, W.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2014, 53, 775.
(g) Shen, X.; Min, Z.; Ni, C.; Zhang, W.; Hu, J. Chem. Sci. 2014, 5, 117.
[3] For some reviews on trifluoromethylation, see:(a) Nie, J.; Guo, H.; Cahard, D.; Ma, J. Chem. Rev. 2011, 111, 455.
(b) Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475.
(c) Studer, A. Angew. Chem., Int. Ed. 2012, 51, 8950.
(d) Furuya, T.;, Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470. For recent examples, see:
(e) Gao, P.; Shen, Y. W.; Fang, R.; Hao, X. H.; Qiu, Z. H.; Yang, F.; Yan, X. B.; Gong, X. J.; Liu, X. Y.; Liang, Y. M. Angew. Chem., Int. Ed. 2014, 53, 7629.
(f) Liu, J. B.; Chen, C.; Chu, L. L.; Qing. F. L. Angew. Chem., Int. Ed. 2015, 54, 11839.
(g) Sahoo, B.; Li, J. L.; Glorius, F. Angew. Chem., Int. Ed. 2015, 54, 11577.
[4] For recent examples, see:(a) Li, L.; Wang, F.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2013, 52, 12390.
(b) Min, Q.; Yin, Z.; Feng, Z.; Guo, W.; Zhang, X. J. Am. Chem. Soc. 2014, 136, 1230.
(c) Feng, Z.; Min, Q.; Xiao, Y.; Zhang, B.; Zhang, X. Angew. Chem., Int. Ed. 2014, 53, 1669.
(d) Wang, X.; Liu, G.; Xu, X.; Shibata, N.; Tokunaga, E.; Shibata, N. Angew. Chem., Int. Ed. 2014, 53, 1827.
(e) Chang, D.; Gu, Y.; Shen, Q. Chem. Eur. J. 2015, 21, 6074.
(f) He, Y. T.; Wang, Q.; Li, L. H.; Liu, X. Y.; Xu, P. F.; Liang, Y. M. Org. Lett. 2015, 17, 5188.
(g) Lin, Q. Y.; Xu, X. H.; Zhang, K.; Qing, F. L. Angew. Chem., Int. Ed. 2016, 55, 1479.
(h) Xie, J.; Zhang, T.; Chen, F. Mehrkens, N.; Rominger, F.; Rudolph, M.; Hashmi, A. S. Angew. Chem., Int. Ed. 2016, 55, 2934.
[5] (a) Blackburn, C. M.; England, D. A.; Kolkmann, F. J. Chem. Soc. Chem. Commun. 1981, 930.
(b) Blackburn, G. M.; Kent, D. E.; Kolkmann, F. J. Chem. Soc., Perkin Trans. 1 1984, 1119.
(c) Erickson, J. A.; Mcloughlin, J. I. J. Org. Chem. 1995, 60, 1626.
(d) Kitazume, T.; Kamazaki, T. Experimental Methods in Organic Fluorine Chemistry, Gordon and Breach Science, Tokyo, 1998.
[6] For recent reviews on photoredox catalysis, see:(a) Xuan, J.; Xiao, W. J. Angew. Chem., Int. Ed. 2012, 51, 6828.
(b) Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527.
(c) Narayanam, J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102.
(d) Tucker, J. W.; Stephenson, C. R. J. J. Org. Chem. 2012, 77, 1617.
(e) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322;
(f) Xi, Y.; Yi, H.; Lei, A. Org. Biomol. Chem. 2013, 11, 2387.
(g) Xie, J.; Jin, H.; Xu, P.; Zhu, C. Tetrahedron Lett. 2014, 55, 36.
[7] (a) Yu, C.; Iqbal, N.; Park, S.; Cho, E. Chem. Commun. 2014, 50, 12884.
(b) Sun, X.; Yu, S. Org. Lett. 2014, 16, 2398.
(c) Su, Y. M.; Hou, Y.; Yin, F.; Xu, Y. M.; Li, Y.; Zheng, X.; Wang, X. S.; Org. Lett. 2014, 16, 2958.
(d) Wang, L.; Wei, X.; Jia, W.; Zhong, J.; Wu. L.; Liu, Q. Org. Lett. 2014, 16, 5842.
(e) Nguyen, J. D.; Tucker, J. W.; Konieczynska, M. D.; Stephenson, C. R. J. J. Am. Chem. Soc. 2011, 133, 4160.
(f) Wallentin, C. J.; Nguyen, J. D.; Finkbeiner. P.; Stephenson, C. R. J. J. Am. Chem. Soc. 2012, 134, 8875.
(g) Jung, J.; Kim, E.; You. Y.; Cho, E. Adv. Synth. Catal. 2014, 356, 2741.
(h) Gu, Z. X.; Zhang, H. L.; Xu, P.; Cheng, Y. X.; Zhu. C. J. Adv. Synth. Catal. 2015, 357, 3057.
(j) Xu, P.; Hu, K. D.; Gu, Z. X.; Cheng, Y. X.; Zhu, C. J. Chem. Commun. 2015, 51, 7222.
[8] (a) Li, W.; Zhu, X.; Mao, H.; Tang, Z.; Cheng, Y.; Zhu, C. Chem. Commun. 2014, 50, 7521.
(b) Qu, C. H.; Xu, P.; Ma, W. J.; Cheng, Y. X.; Zhu, C. J. Chem. Commun. 2015, 51, 13508.
(c) Gu, Z. X.; Zhang, H. L.; Xu, P.; Cheng, Y. X.; Zhu, C. J. Adv. Synth. Catal. 2015, 357, 3057.
(d) Xu, P.; Wang, G. Q.; Zhu, Y. C.; Li, W. P.; Cheng, Y. X.; Li, S. H.; Zhu, C. J. Angew. Chem., Int. Ed. 2016, 55, 2939.
[9] For reviews on aryl migration:(a) Studer, A.; Bossart, M. Tetrahedron 2001, 57, 9649.
(b) Chen, Z. M.; Zhang, X. M.; Tu, Y. Q. Chem. Soc. Rev. 2015, 44, 5220.
[10] For the aryldifluoroacetylation of alkynes reaction, see:Fu, W. J.; Zhu, M.; Zou, G. L.; Xu, C.; Wang, Z. Q.; Ji, B. M. J. Org. Chem. 2015, 80, 4766.
/
| 〈 |
|
〉 |