

可见光促进下氟烷基砜对芳基烯烃的自由基氟烷基化反应
收稿日期: 2016-08-14
修回日期: 2016-09-07
网络出版日期: 2016-09-18
基金资助
项目受973计划(2015CB931900)、国家自然科学基金(21372246,21421002,21472221)、上海市学术带头人计划(15XD1504400)、中国科学院青年创新促进会(2014231)与Syngenta博士生奖学金资助.
Radical Fluoroalkylation of Aryl Alkenes with Fluorinated Sulfones by Visible-Light Photoredox Catalysis
Received date: 2016-08-14
Revised date: 2016-09-07
Online published: 2016-09-18
Supported by
Project supported by the 973 Program (2015CB931900), the National Natural Science Foundation of China (21372246, 21421002, 21472221), Shanghai Aca-demic Research Leader Program (15XD1504400), the Youth Innovation Promotion Association CAS (2014231), and a Syngenta Ph.D. Studentship.
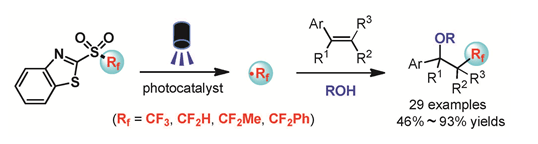
自由基氟烷基化是向有机分子中引入氟烷基的一类非常重要的方法,也是目前有机化学研究的热点之一.近几年来,由于广泛的官能团兼容性和温和的反应条件等优点,可见光促进的氧化还原催化反应得到了长足的发展,已经成为化学键的构建和活化的有力工具.因此,光氧化还原催化的自由基氟烷基化反应,作为向有机化合物中引入氟烷基的有效途径,受到了广泛关注.本文报道了我们发展的氟烷基砜作为一类方便易得的新型氟烷基自由基前体,在可见光氧化还原催化下实现对烯烃的自由基氟烷基化反应.该反应可以高效地向芳基烯烃中引入三氟甲基、二氟甲基、1,1-二氟乙基、苯基二氟甲基等各种含氟烷基基团,并实现对芳基烯烃的双官能团化转化.
荣健 , 倪传法 , 王云泽 , 匡翠文 , 顾玉诚 , 胡金波 . 可见光促进下氟烷基砜对芳基烯烃的自由基氟烷基化反应[J]. 化学学报, 2017 , 75(1) : 105 -109 . DOI: 10.6023/A16080412
The incorporation of fluorine atoms or fluorinated moieties into organic molecules can often lead to significant changes of their physical, chemical, or biological properties. Consequently, fluorinated organic molecules are widely used in areas of pharmaceuticals, agrochemicals and materials. Traditional approaches for the incorporation of fluorinated moieties into organic molecules include nucleophilic, electrophilic, and radical pathways. Among them, radical fluoroalkylations under visible-light photoredox catalysis have attracted much attention because of the mild reaction conditions and broad functional-group tolerance. In our previous work, the radical fluoroalkylation of isocyanides with fluorinated sulfones as the fluoroalkyl radical precursors via Rf-SO2Ar bond cleavage has been achieved under visible-light photoredox catalysis (Rong, J. et al. Angew. Chem., Int. Ed. 2016, 55, 2743). Herein, as a logical extension of our previous research, we report the radical fluoroalkylation of aryl alkenes with fluorinated sulfones as the practical fluoroalkyl radical precursors under visible-light photoredox catalysis. Various fluoroalkyl radicals, including trifluoromethyl (CF3), difluoromethyl (HCF2), 1,1-difluoroethyl (CH3CF2) and (phenyl) difluoromethyl (PhCF2) radicals, can be incorporated into styrene derivatives via this method, delivering the oxyfluoroalkylation products in 46%~93% yields. Typical procedures for this reaction are given as follows:to a Schlenk tube were added 2-vinylnaphthalene (1a) (0.20 mmol, 30.8 mg, 1.0 equiv.), trifluoromethyl 2-benzo[d]thiazolyl sulfone (2b) (0.24 mmol, 64.1 mg, 1.2 equiv.), fac-Ir(ppy)3 (2.7 mg, 0.004 mmol, 2 mol%), H2O (0.5 mL), and acetone (4.5 mL) sequentially. The resulting mixture was degassed with a freeze-pump-thaw procedure (3 times) and irradiated by a 6 W blue LED for 12 h. After the reaction completed, the mixture was extracted with Et2O and dried over anhydrous MgSO4. The organic solvent was removed under reduced pressure and the residue was purified by column chromatography on silica gel by using a 10:1 (V/V) mixture of petroleum ether/EtOAc as an eluent to provide the hydroxytrifluoromethylation product 3a (31.2 mg, 65% yield).

[1] (a) Uneyama, K. Organofluorine Chemistry, Blackwell, Oxford, 2006.
(b) Chambers, D. R. Fluorine in Organic Chemistry, Blackwell, Oxford, 2004.
(c) Kirsch, P. Modern Fluoroorganic Chemistry:Synthesis, Reactivity, Applications, 2nd Ed., Wiley-VCH, Weinheim, 2013.
[2] (a) Qing, F.-L. Chin. J. Org. Chem. 2012, 32, 815. (卿凤翎, 有机化学, 2012, 32, 815.)
(b) Liang, T.; Neumann, C. N.; Ritter, T. Angew. Chem. Int. Ed. 2013, 52, 8214.
(c) Ni, C.; Hu, J. Chem. Soc. Rev. 2016, DOI:10.1039/C6CS00351F.
[3] For recent reviews, see:(a) Koike, T.; Akita, M. Top. Catal. 2014, 57, 967.
(b) Belhomme, M.-C.; Besset, T.; Poisson, T.; Pannecoucke, X. Chem. Eur. J. 2015, 21, 12836.
(c) Ni, C.; Zhu, L.; Hu, J. Acta Chim. Sinica 2015, 73, 90. (倪传法, 朱林桂, 胡金波, 化学学报, 2015, 73, 90.)
(d) Barata-Vallejo, S.; Bonesi, M. S.; Postigo, A. Org. Biomol. Chem. 2015, 13, 11153.
(e) Pan, X.; Xia, H.; Wu, J. Org. Chem. Front. 2016, 3, 1163.
(f) Tan, F.; Xiao, W. Acta Chim. Sinica 2015, 73, 85. (谭芬, 肖文精, 化学学报, 2015, 73, 85.)
[4] (a) Mizuta, S.; Verhoog, S.; Engle, K. M.; Khotavivattana, T.; O'Duill, M.; Wheelhouse, K.; Rassias, G.; Médebielle, M.; Gouverneur, V. J. Am. Chem. Soc. 2013, 135, 2505.
(b) Wilger, D. J.; Gesmundo, N. J.; Nicewicz, D. A. Chem. Sci. 2013, 4, 3160.
(c) Pitre, S. P.; McTiernan, C. D.; Ismaili, H.; Scaiano, J. C. ACS Catal. 2014, 4, 2530.
(d) Yu, B.; Iqbal, N.; Park, S.; Cho, E. J. Chem. Commun. 2014, 50, 12884.
(e) Tang, X.-J.; Zhang, Z.; Dolbier, W. R., Jr. Chem. Eur. J. 2015, 21, 18961.
(f) Lin, Q.-Y.; Xu, X.-H; Zhang, K.; Qing, F.-L. Angew. Chem. Int. Ed. 2016, 55, 1479.
(g) Panferova, L. I.; Tsymbal, A. V.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Org. Lett. 2016, 18, 996.
(h) Zhu, L.; Wang, L.-S.; Li, B.; Fu, B.; Zhang, C.-P.; Li, W. Chem. Commun. 2016, 52, 6371.
(i) Lin, Q.; Chu, L.; Qing, F.-L. Chin. J. Chem. 2013, 31, 885.
[5] (a) Xu, P.; Xie, J.; Xue, Q.; Pan, C.; Cheng, Y.; Zhu, C. Chem. Eur. J. 2013, 19, 14039.
(b) Carboni, A.; Dagousset, G.; Magnier, E.; Masso, G. Org. Lett. 2014, 16, 1240.
(c) Tang, X.-J.; Thomoson, C. S.; Dolbier, W. R., Jr. Org. Lett. 2014, 16, 4594.
(d) Wang, J.-Y.; Su, Y.-M.; Yin, F.; Bao, Y.; Zhang, X.; Xu, Y.-M.; Wang, X.-S. Chem. Commun. 2014, 50, 4108.
(e) Carboni, A.; Dagousset, G.; Magnierb, E.; Masson, G. Chem. Commun. 2014, 50, 14197.
(f) Wang, J.-Y.; Zhang, X.; Bao, Y.; Xu, Y.-M.; Cheng, X.-F.; Wang, X.-S. Org. Biomol. Chem. 2014, 12, 5582.
(g) Gao, F.; Yang, C.; Gao, G.-L.; Zheng, L.; Xia, W. Org. Lett. 2015, 17, 3478.
(h) Thomoson, C. S.; Tang, X.-J.; Dolbier, W. R., Jr. J. Org. Chem. 2015, 80, 1264.
(i) Fu, W.; Zhu, M.; Zou, G.; Xu, C.; Wang, Z.; Ji, B. J. Org. Chem. 2015, 80, 4766.
(j) Zheng, L.; Yang, C.; Xu, Z.; Gao, F.; Xia, W. J. Org. Chem. 2015, 80, 5730.
(k) Song, R.-J.; Liu, Y.; Xie, Y.-X.; Li, J.-H.; Synthesis 2015, 47, 1195.
(l) An, Y.; Li, Y.; Wu. J. Org. Chem. Front. 2016, 3, 570.
[6] (a) 5b.
(b) Yasu, Y.; Koike, T.; Akita, M. Org. Lett. 2013, 15, 2136.
(c) Zhang, Z.; Tang, X.; Thomoson, C. S.; Dolbier, W. R., Jr. Org. Lett. 2015, 17, 3528.
(d) Wei, Q.; Chen, J.-R.; Hu, X.-Q.; Yang, X.-C.; Lu, B.; Xiao, W.-J. Org. Lett. 2015, 17, 4464.
(e) Kim, E.; Choi, S.; Kim, H.; Cho, E. J. Chem. Eur. J. 2013, 19, 6209.
(f) Yu, X.-L.; Chen, J.-R.; Chen, D.-Z.; Xiao, W.-J. Chem. Commun. 2016, 52, 8275.
[7] (a) Yasu, Y.; Koike, T.; Akita, M. Angew. Chem. Int. Ed. 2012, 51, 9567.
(b) 6e.
(c) Wei, X.-J.; Yang, D.-T.; Wang, L.; Song, T.; Wu, L.-Z.; Liu, Q. Org. Lett. 2013, 15, 6054.
(d) Yasu, Y.; Arai, Y.; Tomita, R.; Koike, T.; Akita, M. Org. Lett. 2014, 16, 780.
(e) 5b.
(f) Fu, W.; Zhu, M.; Zou, G.; Xu, C.; Wang, Z. Asian J. Org. Chem. 2014, 3, 1273.
(g) 6d. (h) Deng, Q.-H.; Chen, J.-R.; Wei, Q.; Zhao, Q.-Q.; Lu, L.-Q.; Xiao, W.-J. Chem. Commun. 2015, 51, 3537.
(i) Noto, N.; Miyazawa, K.; Koike, T.; Akita, M. Org. Lett. 2015, 17, 3710.
(j) Arai, Y.; Tomita, R.; Ando, G.; Koike, T.; Akita, M. Chem. Eur. J. 2016, 22, 1262.
(k) Ran, Y.; Lin, Q.-Y.; Xu, X.-H; Qing, F.-L. J. Org. Chem. 2016, 81, 7001.
(l) Noto, N.; Koike, T.; Akita, M. J. Org. Chem. 2016, 81, 7064.
[8] (a) Nguyen, J. D.; Tucker, J. W.; Konieczynska, M. D.; Stephenson, C. R. J. J. Am. Chem. Soc. 2011, 133, 4160.
(b) Wallentin, C.-J.; Nguyen, J. D.; Finkbeiner, P.; Stephenson, C. R. J. J. Am. Chem. Soc. 2012, 134, 8875.
(c) Oh, S. H.; Malpani, Y. R.; Ha, N.; Jung, Y.-S.; Han, S. B. Org. Lett. 2014, 16, 1310.
(d) Tang, X.-J.; Dolbier, W. R., Jr. Angew. Chem. Int. Ed. 2015, 54, 4246.
(e) Bagal, D. B.; Kachkovskyi, G.; Knorn, M.; Rawner, T.; Bhanage, M. B.; Reiser, O. Angew. Chem. Int. Ed. 2015, 54, 6999.
(f) Carboni, A.; Dagousset, G.; Magnier, E.; Masson, G. Synthesis 2015, 47, 2439.
(g) Lin, Q.-Y.; Ran, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2016, 18, 2419.
[9] (a) Prakash, G. K. S.; Hu, J. Acc. Chem. Res. 2007, 40, 921.
(b) Hu, J. J. Fluorine Chem. 2009, 130, 1130.
(c) Zhang, W.; Ni, C.; Hu, J. Top. Curr. Chem. 2012, 308, 25.
(d) Ni, C.; Hu, M.; Hu, J. Chem. Rev. 2015, 115, 765.
[10] Rong, J.; Deng, L.; Tan, P.; Ni, C.; Gu, Y.; Hu, J. Angew. Chem. Int. Ed. 2016, 55, 2743.
/
| 〈 |
|
〉 |