

可见光促进的苄位Csp3-H键活化官能团化反应
收稿日期: 2016-08-16
修回日期: 2016-09-20
网络出版日期: 2016-09-27
基金资助
项目受科技部973计划(No.2012CB725302)、国家自然科学基金(Nos.21390400,21520102003,21272180,21302148)、高等学校博士学科点专项科研基金(No.20120141130002)、教育部长江学者和创新团队发展计划(No.IRT1030)、国家科学技术部基金(No.2012YQ120060)和高等学校学科创新引智计划(111项目)资助.
Visible Light Promoted Benzylic Csp3-H Bond Activation and Functionalization
Received date: 2016-08-16
Revised date: 2016-09-20
Online published: 2016-09-27
Supported by
Project supported by the 973 Program (2012CB725302), the National Natural Science Foundation of China (21390400, 21520102003, 21272180 and 21302148), and the Research Fund for the Doctoral Program of Higher Education of China (20120141130002) and the Program for Changjiang Scholars and Innovative Research Team in University (IRT1030) and the Ministry of Science and Technology of China (2012YQ120060), the Program of Introducing Talents of Discipline to Universities of China (111 Program) is also appreciated.
裴朋昆 , 张凡 , 易红 , 雷爱文 . 可见光促进的苄位Csp3-H键活化官能团化反应[J]. 化学学报, 2017 , 75(1) : 15 -21 . DOI: 10.6023/A16080417
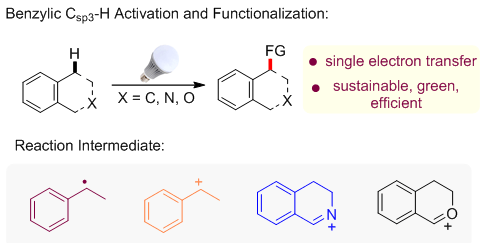
In recent years, visible-light-promoted photoredox catalytic activation of organic molecules has been flourishing vigorously. This kind of methodology usually takes advantage of transition-metal complexes and organic dyes as photosensitizers, which can directly react with organic substrates through a single-electron-transfer (SET) progress under visible light irradiation. It's operable to construct C-X (X=C, N, O …) bond via the radical or radical ion generated during the SET process. On the basis of different key intermediates, this highlight gives a brief summary on the recent development of visible light promoted benzylic Csp3-H activation and functionalization.
[1] (a) Vanjari, R.; Singh, K. N. Chem. Soc. Rev. 2015, 44, 8062;
(b) Zhang, J.; Lu, Q.-Q.; Liu, C.; Lei, A.-W. Chin. J. Org. Chem. 2015, 35(4), 743. (张剑, 陆庆全, 刘超, 雷爱文, 有机化学, 2015, 35(4), 743.);
(c) Huang, Z.; Tang, S.; Lei, A. Sci. Bull. 2015, 60, 1391.
[2] Prier, C. K.; Rankic, D. A.; MacMillan, D. W. Chem. Rev. 2013, 113, 5322.
[3] Koike, T.; Akita, M. Inorg. Chem. Front. 2014, 1, 562.
[4] Ravelli, D.; Protti, S.; Fagnoni, M. Chem. Rev. 2016, 116, 9850.
[5] (a) Xi, Y.; Yi, H.; Lei, A.-W. Org. Biomol. Chem. 2013, 11, 2387;
(b) Zeng, T.-T.; Xuan, J.; Chen, J.-R.; Lu, L.-Q.; Xiao, W.-J. Imaging Science and Photochemistry 2014, 32, 415. (曾婷婷, 宣俊, 陈加荣, 陆良秋, 肖文精, 影像科学与光化学, 2014, 32, 415.)
[6] Hopkinson, M. N.; Sahoo, B.; Li, J.-L.; Glorius, F. Chem. Eur. J. 2014, 20, 3874.
[7] Nicewicz, D. A.; Nguyen, T. M. ACS Catal. 2014, 4, 355.
[8] Hari, D. P.; Konig, B. Angew. Chem. Int. Ed. 2013, 52, 4734.
[9] Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527.
[10] Xuan, J.; Xiao, W.-J. Angew. Chem. Int. Ed. 2012, 51, 6828.
[11] Xia, J.-B.; Zhu, C.; Chen, C. J. Am. Chem. Soc. 2013, 135, 17494.
[12] Xia, J.-B.; Zhu, C.; Chen, C. Chem. Commun. 2014, 50, 11701.
[13] Qvortrup, K.; Rankic, D. A.; MacMillan, D. W. C. J. Am. Chem. Soc. 2014, 136, 626.
[14] Hager, D.; MacMillan, D. W. C. J. Am. Chem. Soc. 2014, 136, 16986.
[15] Rabet, P. T. G.; Fumagalli, G.; Boyd, S.; Greaney, M. F. Org. Lett. 2016, 18, 1646.
[16] Hou, T.-Y.; Lu, P.; Li, P.-X. Tetrahedron Lett. 2016, 57, 2273.
[17] Lu, P.; Hou, T.-Y.; Gu, X.-Y.; Li, P.-X. Org. Lett. 2015, 17, 1954.
[18] Pandey, G.; Laha, R.; Singh, D. J. Org. Chem. 2016, 81, 7161.
[19] Zou, Y.-Q.; Lu, L.-Q.; Fu, L.; Chang, N.-J.; Rong, J.; Chen, J.-R.; Xiao, W.-J. Angew. Chem. Int. Ed. 2011, 50, 7171.
[20] Liu, Q.; Li, Y.-N.; Zhang, H.-H.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Chem. Eur. J. 2012, 18, 620.
[21] Zhong, J.-J.; Meng, Q.-Y.; Wang, G.-X.; Liu, Q.; Chen, B.; Feng, K.; Tung, C.-H.; Wu, L.-Z. Chem. Eur. J. 2013, 19, 6443.
[22] Gao, X.-W.; Meng, Q.-Y.; Li, J.-X.; Zhong, J.-J.; Lei, T.; Li, X.-B.; Tung, C.-H.; Wu, L.-Z. ACS Catal. 2015, 2391.
[23] Zheng, Y. W.; Chen, B.; Ye, P.; Feng, K.; Wang, W.; Meng, Q. Y.; Wu, L.-Z.; Tung, C.-H. J. Am. Chem. Soc. 2016, 138, 10080.
[24] Zhong, J.-J.; Meng, Q.-Y.; Liu, B.; Li, X.-B.; Gao, X.-W.; Lei, T.; Wu, C.-J.; Li, Z.-J.; Tung, C.-H.; Wu, L.-Z. Org. Lett. 2014, 16, 1988.
[25] Zhong, J.-J.; Wu, C.-J.; Meng, Q.-Y.; Gao, X.-W.; Lei, T.; Tung, C.-H.; Wu, L.-Z. Adv. Synth. Catal. 2014, 356, 2846.
[26] Meng, Q.-Y.; Zhong, J.-J.; Liu, Q.; Gao, X.-W.; Zhang, H.-H.; Lei, T.; Li, Z.-J.; Feng, K.; Chen, B.; Tung, C.-H.; Wu, L.-Z. J. Am. Chem. Soc. 2013, 135, 19052.
[27] Ye, P.; Wang, D.-H.; Chen, B.; Meng, Q.-Y.; Tung, C.-H.; Wu, L.-Z. Sci. China. Chem. 2016, 59, 175.
[28] Xiang, M.; Meng, Q.-Y.; Li, J.-X.; Zheng, Y.-W.; Ye, C.; Li, Z.-J.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Chem. Eur. J. 2015, 21, 18080.
[29] Moriyama, K.; Takemura, M.; Togo, H. Org. Lett. 2012, 14, 2414.
[30] Xu, J.; Luo, L.; Xiao, G.; Zhang, Z.; Lin, H.; Wang, X.; Long, J. ACS Catal. 2014, 4, 3302.
[31] Muhldorf, B.; Wolf, R. Chem. Commun. 2015, 51, 8425.
[32] Neu, H. M.; Jung, J.; Baglia, R. A.; Siegler, M. A.; Ohkubo, K.; Fukuzumi, S.; Goldberg, D. P. J. Am. Chem. Soc. 2015, 137, 4614.
[33] Yi, H.; Bian, C.; Hu, X.; Niu, L.; Lei, A. Chem. Commun. 2015, 51, 14046.
/
| 〈 |
|
〉 |