

可见光照射下卤键引发的多氟烷基溴化物与邻二芳基异腈的自由基型插入反应
收稿日期: 2016-09-07
修回日期: 2016-09-26
网络出版日期: 2016-09-27
基金资助
项目受国家自然科学基金面上项目资助(Nos.21672098,21472084,21273102).
Halogen-Bond-Promoted Radical Isocyanide Insertion of o-Diisocyanoarenes with Perfluoroalkyl Bromides under Visible Light Irradiation
Received date: 2016-09-07
Revised date: 2016-09-26
Online published: 2016-09-27
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21672098, 21472084, 21273102).
孙晓阳 , 王文敏 , 马晶 , 俞寿云 . 可见光照射下卤键引发的多氟烷基溴化物与邻二芳基异腈的自由基型插入反应[J]. 化学学报, 2017 , 75(1) : 115 -118 . DOI: 10.6023/A16090480
A halogen-bond-promoted double radical isocyanide insertion of o-diisocyanoarenes with perfluoroalkyl bromides is reported, in which perfluoroalkyl bromides as halogen bond donors and organic bases as halogen bond acceptors. Fluoroalkyl radicals can be generated by a visible-light-induced single electron transfer (SET) process. Fluoroalkyl radicals are trapped by o-diisocyanoarenes to give 2-fluoroalkylated quinoxaline derivatives. These reactions could be carried out under mild conditions with good chemical yields and broad substrate scope. A broad range of fluoroalkyl bromides with different functionalities could undergo this reaction to give the corresponding quinoxaline derivatives in good yields. A variety of o-diisocyanides could be fluoroalkylated to give quinoxalines under our established conditions. The radical nature of this reaction was confirmed by electron paramagnetic resonance (EPR) experiments using tert-butyl-α-phenylnitrone (PBN) as a spin trap. When PBN was introduced into the reaction mixture, a spectrum signal attributed to the spin adduct C8F17-PBN appeared as a triplet of doublets. Without light and amine, almost no signal was observed. These phenomena strongly suggested that the perfluoroalkyl radical was the key intermediate and the generation of the intermediate heavily relied on the presence of light and amine. A series of deuteration experiments were performed and these results suggested that both the amine and solvent could serve as the hydrogen source and solvent was the major source.
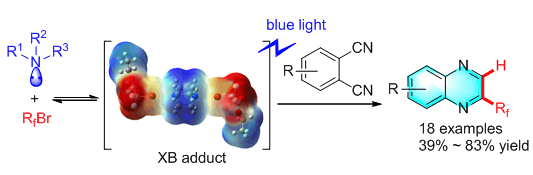
Key words: halogen bond; visible light; isocyanide; fluoroalkylation; quinoxaline; radical
[1] For some reviews on halogen bonds:(a) Metrangolo, P.; Resnati, G. Chem. Eur. J. 2001, 7, 2511.
(b) Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnati, G. Acc. Chem. Res. 2005, 38, 386.
(c) Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Angew. Chem., Int. Ed. 2008, 47, 6114.
(d) Cavallo, G.; Metrangolo, P.; Pilati, T.; Resnati, G.; Sansotera, M.; Terraneo, G. Chem. Soc. Rev. 2010, 39, 3772.
(e) Fourmigué, M. Curr. Opin. Solid State Mater. Sci. 2009, 13, 36.
(f) Legon, A. C. Phys. Chem. Chem. Phys. 2010, 12, 7736.
(g) Lu, Y.; Wang, Y.; Zhu, W. Phys. Chem. Chem. Phys. 2010, 12, 4543.
(h) Erdelyi, M. Chem. Soc. Rev. 2012, 41, 3547.
(i) Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. Chem. Rev. 2016, 116, 2478. For some examples on halogen bonds:
(j) Zhang, X.; Zeng, Y.; Li, X.; Meng, L.; Zheng, S. Acta Chim. Sinica 2009, 67, 593. (张雪英, 曾艳丽, 李晓艳, 孟令鹏, 郑世钧, 化学学报, 2009, 67, 593.)
(k) Zeng, Y.; Ji, L.; Zheng, S.; Meng, L. Acta Chim. Sinica 2011, 69, 1874. (曾艳丽, 吉丽婷, 郑世钧, 孟令鹏, 化学学报, 2011, 69, 1874.)
(l) Fu, Y.; Xiang, Z.; Zhou, J.; Wu, X.; Li, Y.; Jiao, Y. Acta Chim. Sinica 2012, 70, 1847. (付昱, 向子龙, 周军, 吴欣蔚, 李妍, 焦永华, 化学学报, 2012, 70, 1847.).
[2] (a) Pimentel, G. C.; McClella, A. L. Annu. Rev. Phys. Chem. 1971, 22, 347.
(b) Emsley, J. Chem. Soc. Rev. 1980, 9, 91.
(c) Aakeroy, C. B.; Seddon, K. R. Chem. Soc. Rev. 1993, 22, 397.
(d) Perrin, C. L.; Nielson, J. B. Annu. Rev. Phys. Chem. 1997, 48, 511.
(e) Alkorta, I.; Elguero, J. Chem. Soc. Rev. 1998, 27, 163.
(f) Prins, L. J.; Reinhoudt, D. N.; Timmerman, P. Angew. Chem., Int. Ed. 2001, 40, 2382.
(g) Steiner, T. Angew. Chem., Int. Ed. 2002, 41, 48.
(h) Taylor, M. S.; Jacobsen, E. N. Angew. Chem., Int. Ed. 2006, 45, 1520.
(i) Yu, X.; Wang, W. Chem. Asian J. 2008, 3, 516.
(j) Nishio, M.; Umezawa, Y.; Honda, K.; Tsuboyama, S.; Suezawa, H. CrystEngComm 2009, 11, 1757.
(k) Hunt, P. A.; Ashworth, C. R.; Matthews, R. P. Chem. Soc. Rev. 2015, 44, 1257.
[3] (a) Beale, T. M.; Chudzinski, M. G.; Sarwar, M. G.; Taylor, M. S. Chem. Soc. Rev. 2013, 42, 1667.
(b) Desiraju, G. R.; Ho, P. S.; Kloo, L.; Legon, A. C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Pure Appl. Chem. 2013, 85, 1711.
[4] (a) Mukherjee, A.; Tothadi, S.; Desiraju, G. R. Acc. Chem. Res. 2014, 47, 2514.
(b) Berger, G.; Soubhye, J.; Meyer, F. Polym. Chem. 2015, 6, 3559.
[5] (a) Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. Acc. Chem. Res. 2013, 46, 2686.
(b) Gilday, L. C.; Robinson, S. W.; Barendt, T. A.; Langton, M. J.; Mullaney, B. R.; Beer, P. D. Chem. Rev. 2015, 115, 7118.
[6] (a) Caronna, T.; Liantonio, R.; Logothetis, T. A.; Metrangolo, P.; Pilati, T.; Resnati, G. J. Am. Chem. Soc. 2004, 126, 4500.
(b) Nguyen, H. L.; Horton, P. N.; Hursthouse, M. B.; Legon, A. C.; Bruce, D. W. J. Am. Chem. Soc. 2004, 126, 16.
(c) Cariati, E.; Forni, A.; Biella, S.; Metrangolo, P.; Meyer, F.; Resnati, G.; Righetto, S.; Tordin, E.; Ugo, R. Chem. Commun. 2007, 2590.
(d) Lu, Y.; Shi, T.; Wang, Y.; Yang, H.; Yan, X.; Luo, X.; Jiang, H.; Zhu, W. J. Med. Chem. 2009, 52, 2854.
(e) Lu, Y.; Liu, Y.; Xu, Z.; Li, H.; Liu, H.; Zhu, W. Expert Opin. Drug Dis. 2012, 7, 375.
(f) Xu, Z.; Yang, Z.; Liu, Y.; Lu, Y.; Chen, K.; Zhu, W. J. Chem. Inf. Model. 2014, 54, 69.
[7] (a) Bruckmann, A.; Pena, M. A.; Bolm, C. Synlett 2008, 2008, 900.
(b) Bew, S. P.; Fairhurst, S. A.; Hughes, D. L.; Legentil, L.; Liddle, J.; Pesce, P.; Nigudkar, S.; Wilson, M. A. Org. Lett. 2009, 11, 4552.
(c) Dordonne, S.; Crousse, B.; Bonnet-Delpon, D.; Legros, J. Chem. Commun. 2011, 47, 5855.
(d) Walter, S. M.; Kniep, F.; Herdtweck, E.; Huber, S. M. Angew. Chem., Int. Ed. 2011, 50, 7187.
(e) Kniep, F.; Jungbauer, S. H.; Zhang, Q.; Walter, S. M.; Schindler, S.; Schnapperelle, I.; Herdtweck, E.; Huber, S. M. Angew. Chem., Int. Ed. 2013, 52, 7028.
(f) Castelli, R.; Schindler, S.; Walter, S. M.; Kniep, F.; Overkleeft, H. S.; Van der Marel, G. A.; Huber, S. M.; Codée, J. D. C. Chem. Asian J. 2014, 9, 2095.
(g) He, W.; Ge, Y.-C.; Tan, C.-H. Org. Lett. 2014, 16, 3244.
(h) Jungbauer, S. H.; Walter, S. M.; Schindler, S.; Rout, L.; Kniep, F.; Huber, S. M. Chem. Commun. 2014, 50, 6281.
(i) Jungbauer, S. H.; Huber, S. M. J. Am. Chem. Soc. 2015, 137, 12110.
(j) Saito, M.; Tsuji, N.; Kobayashi, Y.; Takemoto, Y. Org. Lett. 2015, 17, 3000.
(k) Sladojevich, F.; McNeill, E.; Börgel, J.; Zheng, S.-L.; Ritter, T. Angew. Chem., Int. Ed. 2015, 54, 3712.
(l) Takeda, Y.; Hisakuni, D.; Lin, C.-H.; Minakata, S. Org. Lett. 2015, 17, 318. For a comprehensive review, see:
(m) Bulfield, D.; Huber, S. M. Chem. Eur. J. 2016, 41, 14434.
[8] (a) Cheng, Y.; Yuan, X.; Ma, J.; Yu, S. Chem. Eur. J. 2015, 21, 8355.
(b) Cheng, Y.; Yu, S. Org. Lett. 2016, 18, 2962.
(c) Sun, X.; Wang, W.; Li, Y.; Ma, J.; Yu, S. Org. Lett. 2016, 18, 4638.
[9] Leifert, D.; Studer, A. Angew. Chem., Int. Ed. 2016, 55, 11660.
[10] The X-ray crystallographic coordinates for structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1476520 (3m') and 1476521 (3n). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
[11] (a) Janzen, E. G.; Blackburn, B. J. J. Am. Chem. Soc. 1968, 90, 5909.
(b) Haire, L. D.; Krygsman, P. H.; Janzen, E. G.; Oehler, U. M. J. Org. Chem. 1988, 53, 4535.
(c) Rehorek, D. Chem. Soc. Rev. 1991, 20, 341.
(d) Zhang, C.-P.; Wang, H.; Klein, A.; Biewer, C.; Stirnat, K.; Yamaguchi, Y.; Xu, L.; Gomez-Benitez, V.; Vicic, D. A. J. Am. Chem. Soc. 2013, 135, 8141.
[12] Julià, L.; Bosch, M. P.; Rodriguez, S.; Guerrero, A. J. Org. Chem. 2000, 65, 5098.
/
| 〈 |
|
〉 |