

可见光驱使下有机染料与镍配合物催化放氢芳构化反应研究
收稿日期: 2016-08-18
修回日期: 2016-11-15
网络出版日期: 2016-11-24
基金资助
项目受国家自然科学基金(Nos.21572090 and 21172102)和中央高校基本科研基金(No.lzujbky-2015-49)资助.
Visible-Light-Driven Aromatization Hydrogen Evolution by Organic Dye and Ni Complex
Received date: 2016-08-18
Revised date: 2016-11-15
Online published: 2016-11-24
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21572090, 21172102) and the Fundamental Research Funds for the Central Universities (No. lzujbky-2015-49).
王孝菊 , 董奎 , 刘强 . 可见光驱使下有机染料与镍配合物催化放氢芳构化反应研究[J]. 化学学报, 2017 , 75(1) : 119 -122 . DOI: 10.6023/A16080421
Pyridine derivatives play an important role in curing and controlling mites, bacteria, weed and so on. Pyrimidine derivatives exist in a number of bioactive natural products, and they have anti-allergy, anti-cancer, anti-inflammatory, insecticidal and some other properties. 3,4-Disubstituted thiophenes not only are important units for the synthesis of natural products, but also serve as key components in some biologically active compounds and material chemistry. In modern society, we have the urgent demand for achieving our products atom economicly and environment-friendly. Under this background, "atom-economy" reactions have been drawing great attention from many chemists and they have got many exciting improvements since then. So, we want to make our own contributions to this area and the following are some of our preliminary results. Our method was based on synergistic application of eosin Y with nickel (II) complex and an external oxidant-free oxidative dehydrogenation aromatization has been developed. At room temperature, Hantzsch 1,4-dihydropyridines, 1,4-dihydropyrimidines, 2,5-dihydrothiophenes and 2,5-dihydropyrroles were transformed into corresponding aromatic compounds in excellent yield under visible light irradiation via hydrogen evolution. We determined the hydrogen with GC-TCD using pure hydrogen as an external standard. It features very mild reaction conditions, high yields and excellent chemo-selectivity. In the previous reports, these transformations usually required higher temperatures and/or stronger oxidizing reagents, resulting in the generation of a large amount of by-products. In addition, the hydrogen evolution reactions were also compared with those of aerobic dehydrogenation. The results indicated that the dehydrogenation aromatizations of hantzsch 1,4-dihydropyridines and 1,4-dihydropyrimidine derivatives under the hydrogen evolution conditions proceeded in higher yields but very low conversions, while the reactions of 2,5-dihydrothiophenes and 2,5-dihydropyrroles gave higher conversions in the aerobic dehydrogenation conditions. So far, this is the first report using organic dye material combined with nickel (II) complexes to achieve dihydrogen dehydrogenation aromatization of heterocyclic compounds.
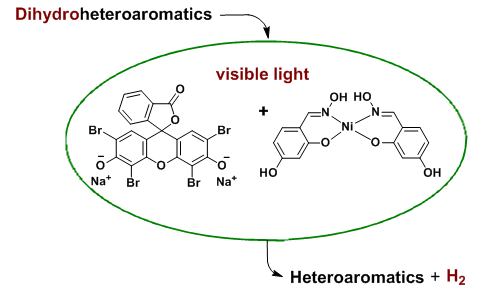
Key words: eosin-Y; nickel complex; hydrogen evolution; aromatization
[1] (a) Li, Y. M.; Jia, F.; Ma, L. N.; Li, Z. P. Acta Chim. Sinica 2015, 73, 1311 (in Chinese). (李远明, 贾凡, 马丽娜, 李志平, 化学学报, 2015, 73, 1311.)
(b) Xu, W. S.; Zhao, S. J.; Bi, X. H.; Liao, P. Q. Chin. J. Org. Chem. 2015, 35, 2095 (in Chinese). (徐文帅, 赵寿经, 毕锡和, 廖沛球, 有机化学, 2015, 35, 2095.)
(c) Kone, J. R.; Marinescu, S. C.; Brunschwig, B. S.; Winkler, J. R.; Gray, H. B. Chem. Sci. 2014, 5, 865.
(d) Li, Q. H.; Huang, R.; Wang, C. J. Acta Chim. Sinica 2014, 72, 830 (in Chinese). (李清华, 黄蓉, 王春江, 化学学报, 2014, 72, 830.)
(e) Dobereiner, G. E.; Crabtree, R. H. Chem. Rev. 2010, 110, 681.
[2] Khadikar, B.; Borkat, S. Synth. Commun. 1998, 28, 207.
[3] Ban, M.; Taquchi, H.; Katsushima, T.; Akoki, S.; Wantanbe, A. Bioorg. Med. Chem. 1998, 6, 1057.
[4] Wright, G. E.; Gombino, J. J. J. Med. Chem. 1984, 27, 181.
[5] Jalander, L. F.; Longquist, J. E. Heterocycles 1998, 48, 743.
[6] Srivastva, S. K.; Agarwal, A.; Murthy, P. K.; Chauhan, P. M. S.; Agarwal, S. K.; Bhaduri, A. P.; Singh, S. N.; Fatima, N.; Chatterjee, R. K. J. Med. Chem. 1999, 42, 1667.
[7] (a) Kappe, C. O. Tetrahedron 1993, 49, 6937.
(b) Kappe, C. O. Acc. Chem. Res. 2000, 33, 879.
(c) Bose, D. S.; Fatima, L.; Mereyala, H. B. J. Org. Chem. 2003, 68, 587.
[8] (a) Gribble, W. G. In Comprehensive Heterocyclic Chemistry, Eds.:Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Pergamon, Oxford, 1996.
(b) Press, J. B. In The Chemistry of Heterocyclic Compounds:Thiophene and Its Derivatives, Ed.:Gronowitz, S., John Wiley & Sons, Inc., New York, 1991.
[9] (a) Roncali, J. Chem. Rev. 1992, 92, 711.
(b) Facchetti, A.; Yoon, M. H.; Marks, T. J. Adv. Mater. 2005, 17, 1705.
(c) Rath, H.; Prabhuraja, V.; Chandrashekar, T. K.; Nag, N.; Goswami, D.; Joshi, B. S. Org. Lett. 2006, 8, 2325.
[10] (a) Jones, R. A.; Bean, G. P. The Chemistry of Pyrroles, Academic Press, London, 1977, p. 1.
(b) Sundberg, R. J. In Comprehensive Heterocyclic Chemistry, Vol. 4, Eds.:Katritzky, A. R.; Rees, C. W., Pergamon Press, Oxford, 1984, p. 370.
(c) Fan, H.; Peng, J.; Hamann, M. T.; Hu, J. F. Chem. Rev. 2008, 108, 264.
[11] (a) Fuerstner, A. Synlett 1999, 1523.
(b) Higgins, S. J. Chem. Soc. Rev. 1997, 26, 247.
(c) McCullough, R. D.; Ewbank, P. C. In Handbook of Conducting Polymers, Eds.:Skotheim, T. A.; Elsenbaumer, R. L.; Reynolds, J. R., Dekker M., New York, 1998, Chapter 9.
[12] (a) Zhu, X. Q.; Zhao, B. J.; Cheng, J. P. J. Org. Chem. 2000, 65, 8158.
(b) Bocker, R. H.; Guengerich, F. P. J. Med. Chem. 1986, 28, 1596.
(c) Ko, K. Y.; Kim, J. Y. Terahedorn Lett. 1999, 40, 3207.
(d) Itoh, T.; Nagata, K.; Matsuya, Y.; Miyazaki, M.; Ohsawa, A. J. Org. Chem. 1997, 62, 3582.
[13] (a) Zhang, G. T.; Hu, X.; Chiang, C. W.; Yi, H.; Pei, P. K.; Singh, A. K.; Lei, A. W. J. Am. Chem. Soc. 2016, 138, 12037.
(b) Zhang, G. T.; Zhang, L. L.; Yi, H.; Luo, Y.; Qi, X. T.; Tung, C. H.; Wu, L. Z.; Lei, A. W. Chem. Commun. 2016, 52, 10407.
(c) Zhang, G.; Liu, C.; Yi, H.; Meng, Q.; Bian, C.; Chen, H.; Jian, J. X.; Wu, L. Z.; Lei, A. W. J. Am. Chem. Soc. 2015, 137, 9273.
(d) McKone, J. R.; Marinescu, S. C.; Brunschwig, B. S.; Winkler, J. R.; Gray, H. B. Chem. Sci. 2014, 5, 865.
(e) Thoi, V. S.; Sun, Y. J.; Long, J. R.; Chang, C. J. Chem. Soc. Rev. 2013, 42, 2388.
(f) Vincent, K. A.; Parkin, A.; Armstrong, F. A. Chem. Rev. 2007, 107, 4366.
[14] (a) Zhang, D.; Wu, L. Z.; Zhou, L.; Han, X.; Yang, Q. Z.; Zhang, L. P.; Tung, C. H. J. Am. Chem. Soc. 2004, 126, 3440.
(b) Wang, D. H.; Peng, M. L.; Han, Y.; Chen, B.; Tung, C. H.; Wu, L. Z. Inorg. Chem. 2009, 49, 9995.
(c) Chen, Y. Z.; Wang, D. H.; Chen, B.; Zhong, J. J.; Tung, C. H.; Wu, L. Z. J. Org. Chem. 2012, 77, 6773.
[15] Xu, Y.; Yin, X.; Huang, Y.; Du, P.; Zhang, B. Chem. Eur. J. 2015, 21, 4571.
[16] (a) Wang, L.; Ma, Z. G.; Wei, X. J.; Meng, Q. Y.; Yang, D. T.; Du, S. F.; Chen, Z. F.; Wu, L. Z.; Liu, Q. Green Chem. 2014, 16, 3752.
(b) Wei, X.; Wang, L.; Jia, W.; Du, S.; Wu, L.; Liu, Q. Chin. J. Chem. 2014, 32, 1245.
/
| 〈 |
|
〉 |