

钠离子电池新型Cu基隧道型氧化物正极材料研究
收稿日期: 2016-08-21
修回日期: 2016-11-10
网络出版日期: 2016-11-24
基金资助
项目受国家自然科学基金(Nos.51222210和11234013)和中国科学院百人计划资助.
Novel Cu Based Oxides with Tunnel Structure as Cathode for Sodium-ion Batteries
Received date: 2016-08-21
Revised date: 2016-11-10
Online published: 2016-11-24
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 51222210 and 11234013), and One Hundred Talent Project of the Chinese Academy of Sciences.
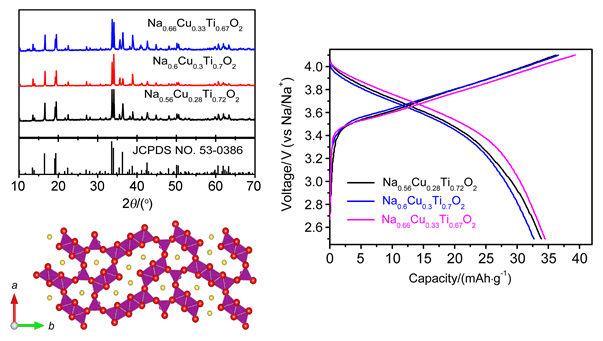
成本较为低廉的钠离子电池成为储能领域的新秀,备受关注.以Na2CO3,CuO,Fe2O3,MnO2和TiO2为原料,通过简单的固相反应法合成了一系列新型Cu基隧道结构化合物.通过X射线衍射(XRD)、扫描电子显微镜(SEM)和电化学性能测试对所得样品的结构、形貌以及电化学性能进行了表征.XRD结果表明材料结构与Na0.44MnO2一样为隧道结构,空间群为pbam.室温下的电化学性能测试表明Mn替代的样品在1.5~4.1 V的范围内表现出了90 mAh/g的比容量,循环稳定,倍率性能较好,1C容量保持率仍为74%.XPS结果验证了Cu在充放电过程中参与了变价.原位XRD结果表明Na0.66Cu0.17Mn0.33Ti0.5O2电极材料在1.5~4.1 V的范围内可以保持稳定的隧道型结构.本工作首次报道了Cu在隧道型结构材料中的变价行为,为进一步设计隧道结构的材料以及钠离子电池正极材料提供新思路.
关键词: Cu3+/Cu2+氧化还原电对; 层状结构; 隧道结构; 钠离子电池
刘丽露 , 戚兴国 , 胡勇胜 , 陈立泉 , 黄学杰 . 钠离子电池新型Cu基隧道型氧化物正极材料研究[J]. 化学学报, 2017 , 75(2) : 218 -224 . DOI: 10.6023/A16080424
Lithium-ion batteries have dominated the electronic and portable device market, since its commercialization in 1990s. However, the cost gets boosted because of the shortage and uneven distribution of lithium. Due to the advantage of cost compared with lithium-ion batteries, sodium-ion batteries are considered as the potential candidates for large scale energy storage systems. Cu based tunnel type materials were first synthesized through simple solid state reaction, with Na2CO3, CuO, Fe2O3, MnO2 and TiO2 as starting materials. These raw materials were weighed and grounded in an agate mortar, followed by heat treatment at 950℃ for 24 h in air. The obtained samples were analyzed by X-ray diffraction (XRD), scanning electron microscopy (SEM) and electrochemical performance test. The XRD results demonstrate the tunnel structure was formed with space group pbam (the same with Na0.44MnO2) for each compound. SEM observation manifests that the distribution of particle size is from several hundred of nanometers to several micrometers. The specifically designed compound with Mn substitution (Na0.66Cu0.17Mn0.33Ti0.50O2) can deliver 90 mAh/g cycled between 1.5~4.1 V. Good cycling stability was verified for this compound, of which 90% of its capacity maintained after 50 cycles at 0.1C rate. Moreover, the rate capability is also good and 74% of its capacity remained when cycled at 1C rate. Charge transfer mechanism was studied by X-ray photoelectron spectroscopy (XPS), and the electroactivity of Cu3+/Cu2+ in this tunnel structure was proved. In addition, we also performed in-situ XRD in order to examine the structure change during sodium extraction and intercalation. Only solid solution reaction took place during the test with shift of peaks or change of the peaks' intensity, however without the appearance of new peaks or disappearance of existed peaks. Here we report, for the first time, the electroactivity of Cu3+/Cu2+ in tunnel type structure. Our results provide new insights in designing tunnel type compound as cathode material for sodium-ion batteries.

[1] Lyu, Z. Y.; Feng, R.; Zhao, J.; Fan, H.; Xu, D.; Wu, Q.; Yang, L. J.; Chen, Q.; Wang, X. Z.; Hu, Z. Acta Chim. Sinica 2015, 73, 1013. (吕之阳, 冯瑞, 赵进, 范豪, 徐丹, 吴强, 杨立军, 陈强, 王喜章, 胡征, 化学学报, 2015, 73, 1013.)
[2] Hua, W.; Wang, Y.; Zhong, Y.; Wang, G.; Zhong, B.; Fang, B.; Guo, X.; Liao, S.; Wang, H. Chin. J. Chem. 2015, 33, 261.
[3] Ou, J.; Yang, L.; Zhang, Y.; Chen, L.; Guo, Y.; Xiao, D. Chin. J. Chem. 2015, 33, 1293.
[4] Armand, M.; Tarascon, J. M. Nature 2008, 451, 652.
[5] Wang, Y. S.; Rong, X. H.; Xu, S. Y.; Hu, Y. S.; Li, H.; Chen, L. Q. Energy Storage Science and Technology 2016, 5, 268. (王跃生, 容晓晖, 徐淑银, 胡勇胜, 李泓, 陈立泉, 储能科学与技术, 2016, 5, 268.)
[6] Pan, H. L.; Hu, Y. S.; Chen, L. Q. Energy Environ. Sci. 2013, 6, 2338.
[7] Li, H.; Wu, C.; Wu, F.; Bai, Y. Acta Chim. Sinica 2014, 72, 21. (李慧, 吴川, 吴锋, 白莹, 化学学报, 2014, 72, 21.)
[8] Xiang, X.; Zhang, K.; Chen, J. Adv. Mater. 2015, 27, 5343.
[9] Wu, D.; Li, X.; Xu, B.; Twu, N.; Liu, L.; Ceder, G. Energy Environ. Sci. 2014, 8, 195.
[10] Hamani, D.; Ati, M.; Tarascon, J.-M.; Rozier, P. Electrochem. Commun. 2011, 13, 938.
[11] Kubota, K.; Ikeuchi, I.; Nakayama, T.; Takei, C.; Yabuuchi, N.; Shiiba, H.; Nakayama, M.; Komaba, S. J. Phys. Chem. C 2015, 119,166.
[12] Li, Y.; Feng, X.; Cui, S.; Shi, Q.; Mi, L.; Chen, W. CrystEngComm 2016, 18, 3136.
[13] Lee, E.; Brown, D. E.; Alp, E. E.; Ren, Y.; Lu, J.; Woo, J.-J.; Johnson, C. S. Chem. Mater. 2015, 27, 6755.
[14] Reddy, B. V. R.; Ravikumar, R.; Nithya, C.; Gopukumar, S. J. Mater. Chem. A 2015, 3, 18059.
[15] Han, M.; Gonzalo, E.; Casas-Cabanas, M.; Rojo, T. J. Power Sources 2014, 258, 266.
[16] Liu, Y.; Fang, X.; Zhang, A.; Shen, C.; Liu, Q.; Enaya, H. A.; Zhou, C. Nano Energy 2016, 27, 27.
[17] Zhu, Y.-E.; Qi, X. G.; Chen, X.; Zhou, X.; Zhang, X.; Wei, J.; Hu, Y.; Zhou, Z. J. Mater. Chem. A 2016, 4, 11103.
[18] Qi, X.; Wang, Y.; Jiang, L.; Mu, L.; Zhao, C.; Liu, L.; Hu, Y.-S.; Chen, L.; Huang, X. Part. Part. Syst. Charact. 2016, 33, 538.
[19] Guo, H.; Wang, Y.; Han, W.; Yu, Z.; Qi, X.; Sun, K.; Hu, Y.-S.; Liu, Y.; Chen, D.; Chen, L. Electrochim. Acta 2015, 158, 258.
[20] Delmas, C.; Fouassier, C.; Hagenmuller, P. Physica B & C 1980, 99, 81.
[21] Doeff, M. M.; Richardson, T. J.; Hollingsworth, J.; Yuan, C. W.; Gonzales, M. J. Power Sources 2002, 112, 294.
[22] Doeff, M. M.; Peng, M. Y.; Ma, Y.; De Jonghe, L. C. J. Electrochem. Soc. 1994, 141, L145.
[23] Parant, J.-P.; Olazcuag, R.; Devalett, M.; Fouassie, C.; Hagenmuller, P. J. Solid State Chem. 1971, 3, 1.
[24] Whitacre, J. F.; Tevar, A.; Sharma, S. Electrochem. Commun. 2010, 12, 463.
[25] Wang, Y.; Liu, J.; Lee, B.; Qiao, R.; Yang, Z.; Xu, S.; Yu, X.; Gu, L.; Hu, Y. S.; Yang, W.; Kang, K.; Li, H.; Yang, X. Q.; Chen, L.; Huang, X. Nat. Commun. 2015, 6, 6401.
[26] Wang, Y.; Mu, L.; Liu, J.; Yang, Z.; Yu, X.; Gu, L.; Hu, Y. S.; Li, H.; Yang, X. Q.; Chen, L.; Huang, X. Adv. Energy Mater. 2015, 5, 1501005.
[27] Xu, S.; Wang, Y.; Ben, L.; Lyu, Y.; Song, N.; Yang, Z.; Li, Y.; Mu, L.; Yang, H.-T.; Gu, L.; Hu, Y.-S.; Li, H.; Cheng, Z.-H.; Chen, L. Huang, X. Adv. Energy Mater. 2015, 5, 1501156.
[28] Wang, J.; Qiu, B.; He, X.; Risthaus, T.; Liu, H.; Stan, M. C.; Schulze, S.; Xia, Y.; Liu, Z.; Winter, M.; Li, J. Chem. Mater. 2015, 27, 4374.
[29] Zhan, P.; Wang, S.; Yuan, Y.; Jiao, K.; Jiao, S. J. Electrochem. Soc. 2015, 162, A1028.
[30] Jiang, X.; Liu, S.; Xu, H.; Chen, L.; Yang, J.; Qian, Y. Chem. Commun. 2015, 51, 8480.
[31] Chu, Q.; Wang, X.; Li, Q.; Liu, X. Acta Crystallogr. Sect. C 2011, 67, i10.
[32] Kim, H.; Kim, D. J.; Seo, D.-H.; Yeom, M. S.; Kang, K.; Kim, D. K. Jung, Y. Chem. Mater. 2012, 24, 1205.
[33] Xu, S. Y.; Wu, X. Y.; Li, Y. M.; Hu, Y. S.; Chen, L. Q. Chin. Phys. B 2014, 23, 118202.
[34] Mu, L.; Xu, S.; Li, Y.; Hu, Y.-S.; Li, H.; Chen, L.; Huang, X. Adv. Mater. 2015, 27, 6928.
[35] Mu, L.; Hu, Y.-S.; Chen, L. Chin. Phys. B 2015, 24, 038202.
[36] Li, Y.; Yang, Z.; Xu, S.; Mu, L.; Gu, L.; Hu, Y.-S.; Li, H.; Chen, L. Adv. Sci. 2015, 2, 1500031.
[37] Li, Y.; Hu, Y.-S.; Qi, X.; Rong, X.; Li, H.; Huang, X.; Chen, L. Energy Storage Mater. 2016, 5, 191.
[38] Sharma, N.; Gonzalo, E.; Pramudita, J. C.; Han, M. H.; Brand, H.; Hart, J. N.; Peng, W. K.; Guo, Z. P.; Rojo, T. Adv. Funct. Mater. 2005, 25, 4994.
/
| 〈 |
|
〉 |