

硫化纳米零价铁去除水体污染物的研究进展
收稿日期: 2017-02-08
网络出版日期: 2017-04-01
基金资助
项目受国家自然科学基金(Nos.51579096,51222805,51521006,51508175)和“万人计划”青年拔尖人才支持计划(2012)资助.
Research Progress of Aqueous Pollutants Removal by Sulfidated Nanoscale Zero-valent Iron
Received date: 2017-02-08
Online published: 2017-04-01
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 51579096, 51222805, 51521006, 51508175) and the National Program for Support of Top-Notch Young Professionals of China (2012).
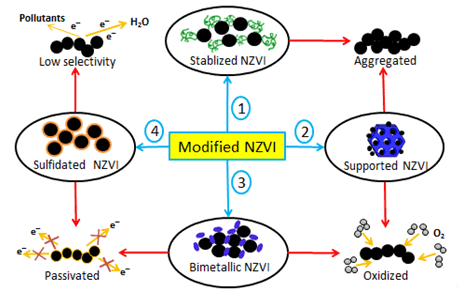
纳米零价铁(NZVI)作为一种原料来源广泛的环境污染修复剂,被广泛应用于地下水和废水等水环境污染修复领域中.尽管这种材料具有反应活性优异、成本低和毒性低的特点,但又面临着自身性质带来的在原位修复和储存等方面的局限.在提高其在水环境中的实际应用潜力的改性方法中,硫化作用成为近10年来的一个研究热点,这也意味着NZVI改性的研究重点从提高NZVI反应活性转移到提高电子的选择性上.硫化型NZVI的制备方法各异,制备方法主要为化学法.这些硫化型材料被大量应用于水体有机污染物降解和重金属去除的研究中来考察它们的实际反应活性,其与水体污染物在不同环境体系下的反应机制也被深入研究.其中,根据硫化型NZVI降解污染物种类和反应条件的不同,大致可以分为吸附、还原和氧化这三大类.尽管目前硫化型NZVI的实际应用仍面临着较多的局限,但是在提高反应活性和电子选择性等方面突破巨大.深入地梳理硫化型NZVI的反应性能和其去除水体中污染物的反应机制的研究进展能够为未来硫化型NZVI的研究指明发展方向.由于硫化型零价铁的优异性能,该材料和相关的硫化铁系材料将会逐步成为环境修复领域的重要材料分支,具有潜在的发展前景.
汤晶 , 汤琳 , 冯浩朋 , 董浩然 , 章毅 , 刘思诗 , 曾光明 . 硫化纳米零价铁去除水体污染物的研究进展[J]. 化学学报, 2017 , 75(6) : 575 -582 . DOI: 10.6023/A17020045
Nanoscale zero-valent iron (NZVI), an environmental remediation agent derived from wide range of raw materials, has been extensively applied in the field of remedying polluted water environment such as groundwater and wastewater. Although NZVI possesses some advantages such as excellent reaction activity, low cost and low toxicity, the limitation of in-situ remediation and storage concerning this kind of material has not been completely overcome yet. Among methods to improve the practical application of NZVI in water environment, sulfidation has become a research hotspot over recent decade. This means that the focus of modifying NZVI has shifted from reaction activity to electron selectivity. Most of the preparation methods of sulfidated NZVI belong to the chemical approach. These sulfidated materials have been heavily used to degrade organic pollutants and remove heavy metals in water to test their practical reactivity. Reaction mechanisms of pollutants and sulfidated NZVI in different environmental systems have also been extensively investigated. Hereinto, according to the species of organic pollutants and the reaction conditions, these reaction mechanisms can be roughly divided into three categories, including adsorption, reduction, and oxidation. In recent years, it is noted that sulfidated NZVI has made great progress to enhance the reaction activity and electrons selectivity, though it still has some limitations in the practical application. It is necessary to thoroughly review recent research progress about the reaction activities of sulfidated NZVI and its reaction mechanisms with pollutants in water, because it can clearly figure out new directions towards future development of sulfidated NZVI application. Due to the superior properties of sulfidated zero-valent iron, this material and relevant iron sulfide-based materials are going to belong to the most important functional materials in the field of environmental remediation with promising development prospect.

[1] Zhang, Y.; Zeng, G.; Tang, L.; Huang, D.; Jiang, X.; Chen, Y. Biosens. Bioelectron. 2007, 22, 2121.
[2] Tang, L.; Zeng, G.; Shen, G.; Li, Y.; Zhang, Y.; Huang, D. Environ. Sci. Technol. 2008, 42, 1207.
[3] Tang, L.; Fang, Y.; Pang, Y.; Zeng, G.; Wang, J.; Zhou, Y.; Deng, Y.; Yang, G.; Cai, Y.; Chen, J. Chem. Eng. J. 2014, 254, 302.
[4] Tang, L.; Yang, G.; Zeng, G.; Ca, Y.; Li, S.; Zhou, Y.; Pang, Y.; Liu, Y.; Zhang, Y.; Luna, B. Chem. Eng. J. 2014, 239, 114.
[5] Li, J.; Qin, H.; Zhang, X.; Guan, X. Acta Chim. Sinica 2017, DOI:10.6023/A17010007. (李锦祥, 秦荷杰, 张雪莹, 关小红, 化学学报, 2017, DOI:10.6023/A17010007.)
[6] Chen, H.; Huang, S.; Zhang, Z.; Liu, Y.; Wang, X. Acta Chim. Sinica 2017, DOI:10.6023/A17010039. (陈海军, 黄舒怡, 张志宾, 刘云海, 王祥科, 化学学报, 2017, DOI:10. 6023/A17010039.)
[7] Zhou, Q.; Li, J.; Wang, M.; Zhao, D. Crit. Rev. Env. Sci. Tec. 2016, 46, 783.
[8] Liang, Z.; Wang, Y. Environ. Prot. 2002, 4, 15. (梁震, 王焰新, 环境保护, 2002, 4, 15.)
[9] Jiemvarangkul, P.; Zhang, W.; Lien, H. L. Chem. Eng. J. 2011, 170, 482.
[10] Phenrat, T.; Saleh, N.; Sirk, K.; Tilton, R. D.; Lowry, G. V. Environ. Sci. Technol. 2007, 41, 284.
[11] Shi, L.; Zhang, X.; Chen, Z. Water Res. 2011, 45, 886.
[12] Greenlee, L. F.; Torrey, J. D.; Amaro, R. L.; Shaw, J. M. Environ. Sci. Technol. 2012, 46, 12913.
[13] Fan, D.; O'Brien Johnson, G.; Tratnyek, P. G.; Johnson, R. L. Environ. Sci. Technol. 2016, 50, 9558.
[14] Fan, D.; O'Carroll, D. M.; Elliott, D. W.; Xiong, Z.; Tratnyek, P. G.; Johnson, R. L.; Garcia, A. N. Remediat. J. 2016, 26, 27.
[15] Ma, X.; Gurung, A.; Deng, Y. Sci. Total Environ. 2013, 443, 844.
[16] El-Temsah, Y. S.; Joner, E. J. Chemosphere 2012, 89, 76.
[17] He, F.; Zhao, D.; Liu, J.; Roberts, C. B. Ind. Eng. Chem. Res. 2007, 46, 29.
[18] Liu, Z.; Zhang, F.; Hoekman, S. K.; Liu, T.; Gai, C.; Peng, N. ACS Sustain. Chem. Eng. 2016, 4, 3261.
[19] Shi, L. N.; Zhang, X.; Chen, Z. L. Water Res. 2011, 45, 886.
[20] Chen, Z.; Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. J. Colloid Interface Sci. 2013, 398, 59.
[21] Wu, Y.; Yang, M.; Hu, S.; Wang, L.; Yao, H. Toxicol. Environ. Chem. 2014, 96, 227.
[22] Krasae, N.; Wantala, K.; Tantriratna, P.; Grisdanurak, N. App. Env. Res. 2014, 36, 15.
[23] Ryu, A.; Jeong, S. W.; Jang, A.; Choi, H. Appl. Catal. B-Environ. 2011, 105, 128.
[24] Xie, Y.; Fang, Z.; Cheng, W.; Tsang, P. E.; Zhao, D. Sci. Total Environ. 2014, 485, 363.
[25] Shih, Y. H.; Chen, M. Y.; Su, Y. F. Appl. Catal. B-Environ. 2011 105, 24.
[26] Kim, E. J.; Kim, J. H.; Azad, A. M.; Chang, Y. S. ACS Appl. Mater. Interfaces 2011, 3, 1457.
[27] Park, S. W.; Kim, S. K.; Kim, J. B.; Choi, S. W.; Inyang, H. I.; Tokunaga, S. Water Air Soil Pollut:Focus. 2006, 6, 97.
[28] He, F. In Iron Environmental Chemistry and Pollution Control Technology Seminar, Shanghai, 2016, p. 41. (何锋, 铁环境化学及污染控制技术研讨会, 上海, 2016, p. 41.)
[29] Yanlai, H.; Weile, Y. Environ. Sci. Technol. 2016, 50, 12992.
[30] Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements, 2nd ed., Elsevier Butterworth Heinemann, 1997.
[31] Kappes, M.; Frankel, G. S.; Sridhar, N.; Carranza, R. M. J. Electrochem. Soc. 2012, 159, C195.
[32] Macdonald, D. D.; Roberts, B.; Hyne, J. B. Corros. Sci. 1978, 18, 411.
[33] Schmitt, G. Corrosion 1991, 47, 285.
[34] Rajajayavel, S. R. C.; Ghoshal, S. Water Res. 2015, 78, 144.
[35] Fan, D.; Anitori, R. P.; Tebo, B. M.; Tratnyek, P. G. Environ. Sci. Technol. 2013, 47, 5302.
[36] Tang, J.; Tang, L.; Feng, H.; Zeng, G.; Dong, H.; Zhang, C.; Huang, B.; Deng Y.; Wang, J.; Zhou, Y. J. Hazard. Mater. 2016, 320, 581.
[37] Butler, E. C.; Hayes, K. F. Environ. Sci. Technol. 2001, 35, 3884.
[38] Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environ. Sci. Technol. 2016, 50, 7290.
[39] Su, Y.; Adeleye, A. S.; Keller, A. A.; Huang, Y.; Dai, C.; Zhou, X.; Zhang, Y. Water Res. 2015, 74, 47.
[40] Xu, C.; Zhang, B.; Wang, Y.; Shao, Q.; Zhou, W.; Fan, D.; Bandstra, J. Z.; Shi, Z.; Tratnyek, P. G. Environ. Sci. Technol. 2016, 50, 11879.
[41] Li, D.; Mao, Z.; Zhong, Y.; Huang, W.; Wu, Y.; Peng, P. Water Res. 2016, 103, 1.
[42] Du, J.; Bao, J.; Lu, C.; Werner, D. Water Res. 2016, 102, 73.
[43] Fan, D.; Anitori, R. P.; Tebo, B. M.; Tratnyek, P. G. Environ. Sci. Technol. 2014, 48, 7409.
[44] Tang, L.; Tang, J.; Zeng, G.; Yang, G.; Xie, X.; Zhou, Y.; Pang, Y.; Fang, Y.; Wang, J.; Xiong, W. Appl. Surf. Sci. 2015, 333, 220.
[45] Ling, X.; Li, J.; Zhu, W.; Zhu, Y.; Sun, X.; Shen, J.; Han, W.; Wang, L. Chemosphere 2012, 87, 655.
[46] Zhuang, Y.; Ahn, S.; Luthy, R. G. Environ. Sci. Technol. 2010, 44, 8236.
[47] Ramos, M. A.; Yan, W.; Li, X. Q.; Koel, B. E.; Zhang, W. X. J. Phys. Chem. C 2009, 113, 14591.
[48] Ai, Z.; Gao, Z.; Zhang, L.; He, W.; Yin, J. J. Environ. Sci. Technol. 2013, 47, 5344.
[49] Wang, L.; Cao, M.; Ai, Z.; Zhang, L. Environ. Sci. Technol. 2014, 48, 3354.
[50] Liu, W.; Ai, Z.; Cao, M.; Zhang, L. Appl. Catal. B-Environ. 2014, 150~151, 1.
[51] Xiong, Z.; Lai, B.; Yang, P.; Zhou, Y.; Wang, J.; Fang, S. J. Hazard. Mater. 2015, 297, 261.
[52] Keenan, C. R.; Sedlak, D. L. Environ. Sci. Technol. 2008, 42, 1262.
[53] Song, S.; Su, Y.; Adeyemi, S. A.; Zhang, Y. Appl. Catal. B-Environ. 2017, 201, 211.
[54] Su, Y.; Adeleye, A. S.; Huang, Y.; Zhou, X.; Keller, A. A.; Zhang, Y. Sci. Rep. 2016, 6, 24358.
[55] Yang, X.; David, M. C. Environ. Sci. Technol. 2010, 44, 8649.
[56] Ariel, N. G.; Hardiljeet, K. B.; Denis, M. O. Environ. Sci. Technol. 2016, 50, 5243.
[57] David, T.; Dimin, F.; Paul, G. T.; Eun-Ju, K.; Yoon-Seok, C. Environ. Sci. Technol. 2012, 46, 12484.
[58] Kim, E. J.; Murugesan, K.; Kim, J. H.; Tratnyek, P. G.; Chang, Y. S. Ind. Eng. Chem. Res. 2013, 52, 9343.
[59] Eun-Ju, K.; Jae-Hwan, K.; Yoon-Seok, C.; David, T.; Paul, G. T. Environ. Sci. Technol. 2014, 48, 4002.
[60] Adeleye, A. S.; Stevenson, L. M.; Su, Y.; Nisbet, R. M.; Zhang, Y.; Keller, A. A. Environ. Sci. Technol. 2016, 50, 5597.
/
| 〈 |
|
〉 |