

生物矿化:构建酸性矿山废水新型被动处理系统的新方法
收稿日期: 2017-02-15
网络出版日期: 2017-04-12
基金资助
项目受国家自然科学基金(Nos.21637003,41371476)资助.
Biomineralization: a Pivotal Process in Developing a Novel Passive Treatment System for Acid Mine Drainage
Received date: 2017-02-15
Online published: 2017-04-12
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21637003, 41371476).
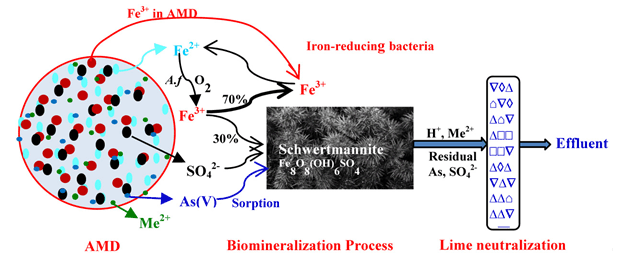
生物矿化是自然界普遍存在的现象,是生物介入下的矿物晶体形成过程,但如何有意识地去调控生物矿化过程并将之用于水处理上报道很少,报告了以生物矿化为核心的含重金属酸性矿山废水(AMD)处理新方法.将嗜酸性氧化亚铁硫杆菌负载在弹性填料上促进酸性矿山废水(AMD)中Fe2+生物氧化并与SO42-形成施威特曼石(schwertmannite)矿物晶核并持续生长,并通过共沉淀和吸附作用原位去除AMD中部分有毒金属.水中残余的未参与生物矿化的Fe3+则在负载有嗜酸性铁还原菌的载体上被还原成Fe2+,继而再通过将嗜酸性氧化亚铁硫杆菌氧化成矿,如此交替构成亚铁生物氧化成矿和残余三价铁生物还原单元,重复多次,最大程度地将AMD中溶解性铁和硫酸根转变为施氏矿物,并去除部分有毒金属,施氏矿物可回收作为新型环境材料.生物矿化后的酸性出水则最后通过极少量的石灰中和即可使水质完全达标.这种"生物矿化-石灰沟渠"新型被动处理系统石灰需求量可减少80%以上,"毒渣"产生量减少90%以上,且"毒渣"中有色金属品位可提高10倍,能用于金属冶炼,是一种极有应用前景的酸性重金属废水处理与资源化新方法.
关键词: 酸性矿山废水; 生物矿化; 重金属; 嗜酸性氧化亚铁硫杆菌; 施氏矿物
周立祥 . 生物矿化:构建酸性矿山废水新型被动处理系统的新方法[J]. 化学学报, 2017 , 75(6) : 552 -559 . DOI: 10.6023/A17020058
Biomineralization, a ubiquitous phenomenon found in nature, is a process of the formation of mineral crystal mediated biologically. However, little information is available on how to consciously regulate and strengthen this biomineralization process with an aim to treat effectively wastewater. Here we develop a novel passive biomineralization-limestone ditch treatment system (PBDTS) for purifying toxic metal-containing acid mine drainages (AMD) generated in many mines. It is well documented that the treatment of AMD by traditional passive limestone ditch treatment system (PLDTS) consume a large amount of lime and consequently produce many toxic residue to be treated due to high concentration of dissolved iron and sulphate in AMD. In the paper, Acidithiobacillus ferrooxidans biofilm formed in elastic filler packed in AMD will oxide Fe2+in AMD into Fe3+ and subsequently form biogenic schwertmannite[Fe8O8(OH)6SO4]. Newly formed schwertmannite as a crystal seed will grow by self and scavenge, to a great extent, most of toxic metalloid As and a few of heavy metal cation through co-precipitation and/or adsorption processes. The remaining non-mineralized Fe3+ is reduced to Fe2+ by Acidiphilic iron-reducing bacterial (Acidiphilium sp.) immobilized in slow-release organic carbon-source material filled in next stage of AMD ditch. The resulting Fe2+ from the biological reduction of Fe3+ are re-oxidized by Acidithiobacillus ferrooxidans and hydrolyzed to form schwertmannite through several oxidizing-reducing cycles. As a result, most of soluble Fe and sulphate in AMD can be removed and recovered in the form of schwertmannite in acidic AMD environment. The effluent of AMD pretreated by biomineralization could be neutralized and easily reach a water quality standard by China only with a few of lime. The results from simulation test showed that such a novel PBDTS could save more than 80% of lime requirement amount and produce less than 10% of toxic neutralized residues with comparison to traditional PLDTS. Moreover, the levels of nonferrous metals in the neutralized residues obtained in PBDTS are more than 10 times higher than that in PLDTS. Therefore, the neutralized residues obtained in PBDTS could be considered as nonferrous metal mine to be exploited. Undoubtedly, more attention should be paid to the role of biomineralization in wastewater treatment. Finally, future research needs are proposed in the paper.

[1] Cui, F. Z. Biomineralization, Tsinghua University Press, Beijing, 2007, pp. 1~85. (崔福斋, 生物矿化, 清华大学出版社, 北京, 2007, pp. 1~85.)
[2] Yao, J.-M.; Wei, K.-M.; Li, L.; Kong, X.-D.; Zhu, Y.-Q.; Lin, F. Acta Chim. Sinica 2007, 65, 635. (姚菊明, 魏克民, 励丽, 孔祥东, 祝永强, 林凤, 化学学报, 2007, 65, 635.)
[3] Zhang, S.; Yang, R.; Ouyang, J. Acta Chim. Sinica 2011, 69, 284. (张生, 杨如娥, 欧阳健明, 化学学报, 2011, 69, 284.)
[4] Wang, L.-J.; Wang, Y.-H.; Zhang, F.-S.; Yang, W.-S.; Li, T.-J. Acta Chim. Sinica 2002, 60, 1144. (王荔军, 王运华, 张福锁, 杨文胜, 李铁津, 化学学报, 2002, 60, 1144.)
[5] Yu, R.-L.; Zhong, D.-L.; Miao, L.; Wu, F.-D.; Qiu, G.-Z.; Gu, G.-H. Trans. Nonferrous Met. Soc. China 2011, 21, 1634.
[6] Liang, C.-L.; Xia, J.-L.; Yang, Y.; Nie, Z.-Y.; Qiu, G.-Z. Chin. J. Nonferrous Met. 2012, 22, 265. (梁长利, 夏金兰, 杨益, 聂珍媛, 邱冠周, 中国有色金属学报, 2012, 22, 265.)
[7] Bigham, J. M.; Schwertmann, U.; Murad, E.; Carlson, L. Geochim. Cosmochim. Acta 1990, 54, 2743.
[8] Bigham, J. M.; Carlson, L.; Murad, E. Mineral Mag. 1994, 58, 641.
[9] Liao, Y. H.; Zhou, L. X.; Bai, S. Y.; Liang, J. R.; Wang, S. M. Appl. Geochem. 2009, 24, 1739.
[10] Singer, P. C.; Stumm, W. Science 1970, 167, 1121.
[11] España, J. S.; Pamo, E. L.; Santofimia, E.; Aduvire, O.; Reyes, L.; Barettino, D. Appl. Geochem. 2005, 20, 1320.
[12] Myers, T. J. Hydrol. 2016, 533, 277.
[13] He, G. H.; Cao, C. China Economic Weekly 2009, 16, 16. (贺广华, 曹昌, 中国经济周刊, 2009, 16, 16.)
[14] Dang, Z. Source Control of Mine Pollution, Science Press, Beijing, 2015, pp. 5~9. (党志, 矿区污染源头控制, 科学出版社, 北京, 2015, pp. 5~9.)
[15] Liao, J. B.; Wen, Z. W.; Ru, X.; Chen, J. D.; Wu, H. Z.; Wei, C. H. Ecotox. Environ. Safe. 2016, 124, 460.
[16] Chen, M.; Lu, G.; Guo, C.; Yang, C.; Wu, J.; Huang, W.; Yee, N.; Dang, Z. Chemosphere 2015, 119, 734.
[17] Gammons, C. H.; Duaime, T. E.; Parker, S. R.; Poulson, S. R.; Kennelly, P. Chem. Geol. 2010, 269, 100.
[18] Qureshi, A.; Maurice, C.; Öhlander, B. J. Geochem. Explor. 2016, 160, 44.
[19] VanGeen, A.; Adkins, J. F.; Boyle, E. A.; Nelson, C. H.; Palanques, A. Geology 1997, 25, 291.
[20] Maicaneanu, A.; Bedelean, H.; Ardelean, M.; Burca, S.; Stance, M. Chemosphere 2013, 93, 1400.
[21] Papassiopi, N.; Zaharia, C.; Xenidis, A.; Adam, K.; Liakopoulos, A.; Romaidis, I. Miner. Eng. 2014, 64, 78.
[22] Caraballo, M. A.; Macías, F.; Nieto, J. M.; Ayora, C. Sci. Total Environ. 2016, 539, 427.
[23] Johnson, D. B.; Hallberg, K. B. Sci. Total Environ. 2005, 338, 3.
[24] Akcil, A.; Koldas, S. J. Clean. Prod. 2006, 14, 1139.
[25] Gibert, O.; J. De Pablo, J.; Cortina, J. L.; Ayora, C. Rev. Environ. Sci. Biotech. 2002, 1, 327.
[26] Sánchez-Andrea, I.; Sanz, J. L.; Bijmans, M. F. M.; Stams, A. J. M. J. Hazard. Mater. 2014, 269, 98.
[27] Kalin, M.; Fyson, A.; Wheeler, W. N. Sci. Total Environ. 2006, 366, 395.
[28] Shim, M. J.; Choi, B. Y.; Lee, G.; Hwang, Y. H.; Yang, J. S.; O'Loughlin, E. J.; Kwon, M. J. J. Geochem. Explor. 2015, 159, 234.
[29] Iakovleva, E.; Makila, E.; Salonen, J.; Sitarz, M.; Wang, S. B.; Sillanpaa, M. Ecol. Eng. 2015, 81, 30.
[30] Kleinmann, R. L. P.; Hedin, R. S. Pollut. Eng. 1993, 25, 20.
[31] Santomartino, S.; Webb, J. A. Appl. Geochem. 2007, 22, 2344.
[32] Yang, Z.; Zhu, L.; Liu, R.; Qu, J. Chin. J. Environ. Eng. 2014, 8, 2205. (杨中超, 朱利军, 刘锐平, 曲久辉, 环境工程学报, 2014, 8, 2205.)
[33] Schwertmann, U; Bigham, J. M.; Murad, E. Eur. J. Mineral. 1995, 7, 547.
[34] Xie, Y. Ph. D. Dissertation, Nanjing Agricultural University, Nanjing, 2010 (in Chinese). (谢越, 博士论文, 南京农业大学, 南京, 2010.)
[35] Fukushi, K.; Sasaki, M.; Sato, T.; Yanase, N.; Amano, H.; Ikeda, H. Appl. Geochem. 2003, 18, 1267.
[36] Loan, M.; Richmond, W. R.; Parkinson, G. M. J. Cryst. Growth. 2005, 275, 1875.
[37] Li, Z. Y.; Liang, J. R.; Bai, S. Y.; Zhou, L. X. Acta Scientiae Circumstantiae 2011, 31, 460. (李浙英, 梁剑茹, 柏双友, 周立祥, 环境科学学报, 2011, 31, 460.)
[38] Liu, F. W.; Zhou, J.; Zhang, S. S.; Liu, L. L.; Zhou, L. X.; Fan, W. H. PLoS ONE 2015, 10, e0138891.
[39] Bigham, J. M.; Schwertmann, U.; Pfab, G. Appl. Geochem. 1996, 11, 845.
[40] Jambor, J. L.; Dutrizac, J. E. Chem. Rev. 1998, 98, 2549.
[41] Gramp, J. P.; Jones, F. S.; Bigham, J. M.; Tuovinen, O. H. Hydrometallurgy 2008, 94, 29.
[42] Bai, S. Y.; Xu, Z. H.; Wang, M.; Liao, Y. H.; Liang, J. R; Zheng, C. C.; Zhou, L. X. Mater. Sci. Eng. C 2012, 32, 2323.
[43] Regenspurg, S.; Peiffer, S. Appl. Geochem. 2005, 20, 1226.
[44] Liao, Y. H.; Liang, J. R.; Zhou, L. X. Chemosphere 2011, 83, 295.
[45] Barham, R. J. Mater. Res. 1997,12, 2751
[46] Liao, Y. H.; Zhou, L. X.; Liang, J. R.; Xiong, H. X. Mater. Sci. Eng. C 2009, 29, 211.
[47] Bai, S. Y. Ph. D. Dissertation, Nanjing Agricultural University, Nanjing, 2012. (柏双友, 博士论文, 南京农业大学, 南京, 2012.)
[48] Wang, N. Ph. D. Dissertation, Nanjing Agricultural University, Nanjing, 2016. (王宁, 博士论文, 南京农业大学, 南京, 2016.)
[49] Majzlan, J.; Navrotsky, A.; Schwertmann, U. Geochim. Cosmochim. Acta 2004, 68, 1049.
[50] Hohenberg, P. C.; Halperin, B. I. Rev. Mod. Phys. 1977, 49, 435.
[51] Tang, R. K.; Wang, L. J.; Orme, C. A.; Bonstein, T.; Bush, P. J.; Nancollas, G. H. Angrew. Chem., Int. Ed. 2004, 43, 2697.
[52] Wang, M.; Zhou, L. X. Hydrometallurgy 2012, 125, 152.
[53] Liu, F. W.; Zhou, J.; Zhou, L. X.; Zhang, S. S.; Liu, L. L.; Wang, M. J. Hazard. Mater. 2015, 299, 404.
[54] Hou, Q. J.; Fang, D.; Liang, J. R.; Zhou, L. X. Plos One 2015, 10, e0120966.
[55] Ahoranta, S. H.; Kokko, M. E.; Papirio, S.; Özkaya, B.; Puhakka, J. S. J. Hazard. Mater. 2016, 306, 124.
[56] Xie, Y.; Zhou, L. X. Pedosphere 2013, 23, 402.
[57] Qu, J. H.; Wang, K. J.; Wang, H. C.; Yu, G.; Ke, B.; Yu, H. Q. China Environ. News 2014, 1, 7. (曲久辉, 王凯军, 王洪臣, 柯兵, 俞汉青, 中国环境报, 2014, 1, 7.)
[58] Huang, X. Water Wastewater Eng. 2013, 39, 1. (黄霞, 给水排水, 2013, 39, 1.)
[59] Zhou, L. X. Earth Science Frontiers 2008, 15, 74. (周立祥, 地学前缘, 2008, 15, 74.)
/
| 〈 |
|
〉 |