

铜催化苯乙烯不对称硼胺化反应
收稿日期: 2017-04-12
网络出版日期: 2017-05-09
基金资助
项目受国家自然科学基金(Nos.21472184,21572218和21402186)资助.
Copper-Catalyzed Enantioselective Aminoboration of Styrenes with Chiral Sulfoxide Phosphine Ligand
Received date: 2017-04-12
Online published: 2017-05-09
Supported by
Project supported by the National Natural Science Foundation of China (Nos.21472184,21572218 and 21402186).
张涌灵 , 王敏 , 曹鹏 , 廖建 . 铜催化苯乙烯不对称硼胺化反应[J]. 化学学报, 2017 , 75(8) : 794 -797 . DOI: 10.6023/A17040144
To date, copper catalysis has become an attractive approach to access multifunctional alkylborons through borylative coupling processes, many important protocols such as carboboration, stannylboration and aminoboration were developed. Among these methods, however, there is no report involving enantioselective aminoboration of simple styrene substrates, which can generate a class of useful chiral compounds. In this work, an enantioselective Cu-catalyzed aminoboration of styrenes by using a chiral sulfoxide-phosphine (SOP) ligand was developed, chiral β-aminoalkylboranes were obtained in satisfied yields and ee values, and these products can be readily converted to a class of valuable β-hydroxylalkylamines. A general procedure for the aminoboration of styrenes is as following:in glove box, CuCl (0.02 mmol), chiral sulfoxide phosphine L1 (0.022 mmol) and 2.0 mL of dried tetrahydrofuran were added into a flame-dried tube, the resolved solution was stirred for 30 min at room temperature, then bis(pinacolato)diboron (B2pin2) (0.3 mmol), t-BuOLi (0.6 mmol) and styrene (0.2 mmol) were added. The tube was taken out of the glove box and cooled to 0℃. Electrophilic amination reagent, O-benzoyl-N,N-dibenzylhydroxylamine (2a, 0.3 mmol), was dissolved in 1.0 mL of ethyl acetate and added to the mixture, the resolved mixture was stirred at 0℃ for 24 h. The crude product was filtered through a celite pad, concentrated and oxidized by NaBO3·4H2O. The mixture was extracted three times with ethyl acetate, concentrated and purified with silica gel chromatography to give the desired β-hydroxylalkylamines, the enantioselective excess of products were determined by chiral HPLC analysis. Broad substrate scope which related to steric and electronic effect were compatible in this catalysis under the standard conditions. To demonstrate the utility of this method, a gram scale experiment was performed and the desired product was obtained in 92% isolated yield and 90% ee. The benzyl group of products can be readily removed via a Pd/C-catalyzed hydrogenation process and the corresponding product with a free amino group in excellent yield (95%).
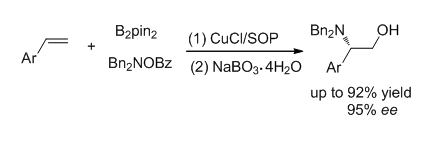
[1] (a) Pelter, A.; Smith, K.; Brown, H. C. Borane Reagents, Academic Press, London, 1988;
(b) Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457;
(c) Davison, M.; Hughes, A. K.; Marder, T. B.; Wade, K. Contemporary Boron Chemistry, RSC, Cambridge, U. K., 2000;
(d) Boronic Acids, 2nd ed.; Ed.:Hall, D. G., Wiley-VCH, Weinheim, Germany, 2011.
[2] (a) Ishiyama, T.; Matsuda, N.; Miyaura, N.; Suzuki, A. J. Am. Chem. Soc. 1993, 115, 11018;
(b) Ishiyama, T.; Matsuda, N.; Murata, M.; Ozawa, F.; Suzuki, A.; Miyaura, N. Organometallics 1996, 15, 713;
(c) Lesley, G.; Nguyen, P.; Taylor, N. J.; Marder, T. B. Organometallics 1996, 15, 5137;
(d) Ishiyama, T.; Yamamoto, M.; Miyaura, N. Chem. Commun. 1996, 2073;
(e) Ishiyama, T.; Yamamoto, M.; Miyaura, N. Chem. Commun. 1997, 689;
(f) Thomas, R. L.; Souza, F. E. S.; Marder, T. B. J. Chem. Soc., Dalton Trans. 2001, 1650;
(g) Yang, F.-Y.; Cheng, C.-H. J. Am. Chem. Soc. 2001, 123, 761;
(h) Burks, H. E.; Kliman, L. T.; Morken, J. P. J. Am. Chem. Soc. 2009, 131, 9134;
(i) Kliman, L. T.; Mlynarski, S. N.; Morken, J. P. J. Am. Chem. Soc. 2009, 131, 13210;
(j) Iwadate, N.; Suginome, M. J. Am. Chem. Soc. 2010, 132, 2548;
(k) Coombs, J. R.; Haeffner, F.; Kliman, L. T.; Morken, J. P. J. Am. Chem. Soc. 2013, 135, 11222;
(l) Coombs, J. R.; Zhang, L.; Morken, J. P. J. Am. Chem. Soc. 2014, 136, 16140; For review, see:Ishiyama, T.; Ishida, K.; Miyaura, N. Tetrahedron 2001, 57, 9813 and references therein.
[3] Selected examples, see:(a) Ito, H.; Yamanaka, H.; Tateiwa, J.-i.; Hosomi, A. Tetrahedron Lett. 2000, 41, 6821;
(b) Zhu, W.; Ma, D. Org. Lett. 2006, 8, 261;
(c) Beenen, M. A.; An, C.; Ellman, J. A. J. Am. Chem. Soc. 2008, 130, 6910;
(d) Lee, J. E.; Yun, J. Angew. Chem., Int. Ed. 2008, 47, 145;
(e) Lipshutz, B. H.; Boskovic, Z. V.; Aue, D. H. Angew. Chem., Int. Ed. 2008, 47, 10183;
(f) Chen, I. H.; Yin, L.; Itano, W.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2009, 131, 11664;
(g) Kleeberg, C.; Dang, L.; Lin, Z.; Marder, T. B. Angew. Chem., Int. Ed. 2009, 48, 5350;
(h) Lillo, V.; Prieto, A.; Bonet, A.; Díaz-Requejo, M. M.; Ramírez, J. s.; Pérez, P. J.; Fernández, E. Organometallics 2009, 28, 659;
(i) Noh, D.; Chea, H.; Ju, J.; Yun, J. Angew. Chem., Int. Ed. 2009, 48, 6062;
(j) O'Brien, J. M.; Lee, K. S.; Hoveyda, A. H. J. Am. Chem. Soc. 2010, 132, 10630;
(k) Lee, J. C.; McDonald, R.; Hall, D. G. Nat. Chem. 2011, 3, 894;
(l) Moure, A. L.; Arrayas, R. G.; Carretero, J. C. Chem. Commun. 2011, 47, 6701;
(m) Solé, C.; Whiting, A.; Gulyás, H.; Fernández, E. Adv. Synth. Catal. 2011, 353, 376;
(n) Burns, A. R.; Solana Gonzalez, J.; Lam, H. W. Angew. Chem., Int. Ed. 2012, 51, 10827;
(o) Ito, H.; Kubota, K. Org. Lett. 2012, 14, 890;
(p) Yang, C. T.; Zhang, Z. Q.; Tajuddin, H.; Wu, C. C.; Liang, J.; Liu, J. H.; Fu, Y.; Czyzewska, M.; Steel, P. G.; Marder, T. B.; Liu, L. Angew. Chem., Int. Ed.. 2012, 51, 528;
(q) Feng, X.; Jeon, H.; Yun, J. Angew. Chem., Int. Ed. 2013, 52, 3989;
(r) Semba, K.; Nakao, Y. J. Am. Chem. Soc. 2014, 136, 7567;
(s) Smith, K. B.; Logan, K. M.; You, W.; Brown, M. K. Chem. Eur. J. 2014, 20, 12032;
(t) Logan, K. M.; Smith, K. B.; Brown, M. K. Angew. Chem., Int. Ed. 2015, 54, 5228;
(u) Su, W.; Gong, T. J.; Lu, X.; Xu, M. Y.; Yu, C. G.; Xu, Z. Y.; Yu, H. Z.; Xiao, B.; Fu, Y. Angew. Chem., Int. Ed. 2015, 54, 12957;
(v) Liu, Q.; Tian, B.; Tian, P.; Tong, X.; Lin, G.-Q. Chin. J. Org. Chem. 2015, 35, 1; (刘强, 田兵, 田平, 童晓峰, 林国强, 有机化学, 2015, 35, 1.)
(w) Semba, K.; Ohtagaki, Y.; Nakao, Y. Org. Lett. 2016, 18, 3956;
(x) Smith, J. J.; Best, D.; Lam, H. W. Chem. Commun. 2016, 52, 3770;
(y) Logan, K. M.; Brown, M. K. Angew. Chem., Int. Ed. 2017, 56, 851.
[4] Selected examples, see:(a) Adams, C. J.; Baber, R. A.; Batsanov, A. S.; Bramham, G.; Charmant, J. P.; Haddow, M. F.; Howard, J. A.; Lam, W. H.; Lin, Z.; Marder, T. B.; Norman, N. C.; Orpen, A. G. Dalton Trans. 2006, 1370;
(b) Obligacion, J. V.; Chirik, P. J. Org. Lett. 2013, 15, 2680;
(c) Obligacion, J. V.; Chirik, P. J. J. Am. Chem. Soc. 2013, 135, 19107.
(d) Zhang, L.; Peng, D.; Leng, X.; Huang, Z. Angew. Chem., Int. Ed. 2013, 52, 3676;
(e) Cao, Y.; Zhang, Y.; Zhang, L.; Zhang, D.; Leng, X.; Huang, Z. Org. Chem. Front. 2014, 1, 1101;
(f) Chen, J.; Xi, T.; Lu, Z. Org. Lett. 2014, 16, 6452;
(g) Chen, J.; Xi, T.; Ren, X.; Cheng, B.; Guo, J.; Lu, Z. Org. Chem. Front. 2014, 1, 1306;
(h) Zhang, L.; Zuo, Z.; Leng, X.; Huang, Z. Angew. Chem., Int. Ed. 2014, 53, 2696;
(i) Zhang, L.; Zuo, Z.; Wan, X.; Huang, Z. J. Am. Chem. Soc. 2014, 136, 15501;
(j) Guo, J.; Chen, J.; Lu, Z. Chem. Commun. 2015, 51, 5725;
(k) Zhang, L.; Huang, Z. J. Am. Chem. Soc. 2015, 137, 15600;
(l) Zhang, H.; Lu, Z. ACS Catal. 2016, 6, 6596;
(m) Zuo, Z.; Huang, Z. Org. Chem. Front. 2016, 3, 434;
(n) Zuo, Z.; Yang, J.; Huang, Z. Angew. Chem., Int. Ed. 2016, 55, 10839;
(o) Xi, T.; Lu, Z. ACS Catal. 2017, 7, 1181.
[5] (a) Suginome, M.; Matsuda, T.; Yoshimoto, T.; Ito, Y. Org. Lett. 1999, 1, 1567;
(b) Suginome, M.; Shirakura, M.; Yamamoto, A. J. Am. Chem. Soc. 2006, 128, 14438;
(c) Ely, R. J.; Morken, J. P. J. Am. Chem. Soc. 2010, 132, 2534.
[6] Reviews:(a) Semba, K.; Fujihara, T.; Terao, J.; Tsuji, Y. Tetrahedron 2015, 71, 2183.
(b) Liu, Y.; Zhang, W. Chin. J. Org. Chem. 2016, 36, 2249. (刘媛媛, 张万斌, 有机化学, 2016, 36, 2249.)
[7] (a) Bloch, R. Chem. Rev. 1998, 98, 1407;
(b) Ramadhar, T. R.; Batey, R. A. Synthesis 2011, 1321;
(c) Yus, M.; González-Gómez, J. C.; Foubelo, F. Chem. Rev. 2013, 113, 5595.
[8] (a) Matsuda, N.; Hirano, K.; Satoh, T.; Miura, M. J. Am. Chem. Soc. 2013, 135, 4934;
(b) Sakae, R.; Matsuda, N.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2014, 16, 1228;
(c) Parra, A.; Amenos, L.; Guisan-Ceinos, M.; Lopez, A.; Garcia Ruano, J. L.; Tortosa, M. J. Am. Chem. Soc. 2014, 136, 15833;
(d) Sakae, R.; Hirano, K.; Miura, M. J. Am. Chem. Soc. 2015, 137, 6460;
(e) Sakae, R.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2015, 54, 613;
(f) Kato, K.; Hirano, K.; Miura, M. Angew. Chem., Int. Ed. 2016, 55, 14400;
(g) Nishikawa, D.; Hirano, K.; Miura, M. Org. Lett. 2016, 18, 4856;
(h) Shi, M. Chem. Commun. 2016, 52, 5273.
[9] (a) Chen, B.; Cao, P.; Yin, X.; Liao, Y.; Jiang, L.; Ye, J.; Wang, M.; Liao, J. ACS Catal. 2017, 7, 2425;
(b) Jia, T.; Cao, P.; Wang, B.; Lou, Y.; Yin, X.; Wang, M.; Liao, J. J. Am. Chem. Soc. 2015, 137, 13760;
(c) Jia, T.; Cao, P.; Wang, D.; Lou, Y.; Liao, J. Chem. Eur. J. 2015, 21, 4918.
[10] Metro, T. X.; Appenzeller, J.; Pardo, D. G.; Cossy, J. Org. Lett. 2006, 8, 3509.
/
| 〈 |
|
〉 |