

靛红衍生的硝酮与酮亚胺的不对称直接氮杂插烯Mannich反应
Direct Asymmetric Aza-Vinylogous Mannich Reaction of Nitrones from Isatins and Ketimines
Received date: 2017-06-26
Online published: 2017-09-04
Supported by
Project supported by the National Natural Science Foundation of China (No. 21602143).
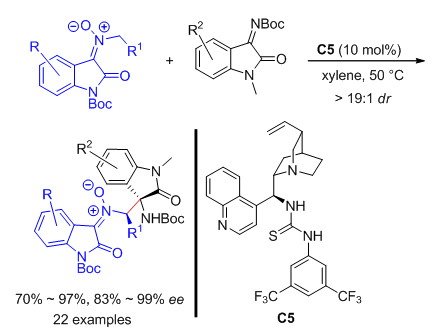
不对称直接插烯Mannich反应是一类高效构建手性δ-氨基-α,β-不饱和羰基化合物的方法,但这类反应主要局限于以γ-丁烯酸内酯及类似物和α,α-二氰基烯烃等作为亲核试剂前体,因此发展新的插烯亲核试剂尤为重要.本工作报道了一类从靛红衍生且含N-CH结构的硝酮化合物,由于氧化吲哚骨架的强吸电子效应能在温和碱性条件下生成氮杂二烯醇中间体,高效与靛红衍生的亚胺发生直接氮杂插烯Mannich反应.采用金鸡纳碱衍生的手性双功能叔胺硫脲催化剂,以高收率(70%~97%)、高立体选择性(83%~99% ee,>19∶1 dr)合成富官能团化并含相邻季碳-叔碳手性中心的硝酮化合物,且可进一步与缺电烯烃发生[3+2]偶极环加成反应构建含有氢化异噁唑环的吲哚螺环复杂骨架.这类靛红衍生的硝酮作为氮杂插烯亲核试剂可能在不对称合成中具有更为广阔的应用.
关键词: 不对称催化; 有机催化; 插烯Mannich反应; 硝酮; 酮亚胺
石明林 , 詹固 , 杜玮 , 陈应春 . 靛红衍生的硝酮与酮亚胺的不对称直接氮杂插烯Mannich反应[J]. 化学学报, 2017 , 75(10) : 998 -1002 . DOI: 10.6023/A17060277
Direct asymmetric vinylogous Mannich reaction is an efficient and powerful method for the synthesis of δ-amino-α,β-unsaturated carbonyl compounds; however, the nucleophiles are generally limited to γ-butenolides and α,α-dicyanoolefins. Therefore, it is highly desirable to design new vinylogous nucleophiles and develop the related asymmetric reactions. Recently, we disclosed a new type of nitrones derived from isatins and N-benzyl hydroxylamines, which could easily generate nitrone ylide species in the presence of a tertiary amine, and undergo asymmetric formal[3+2] cycloadditions with α,β-unsaturated aldehydes via iminium ion catalysis of a chiral secondary amine. Subsequently, we found that such nitrone ylides could isomerize to more interesting aza-dienolate-type intermediates, and engage in direct stereoselective aza-vinylogous Michael reactions with nitroalkenes under the catalysis of a bifunctional thiourea-tertiary amine, delivering chiral nitrone derivatives with extended carbon skeletons without subsequent cyclization. In this case, the same type of nitrones are employed as nucleophilic precursors under the catalysis of a cinchona alkaloid-based thiourea substance, and effectively assembled with isatins-derived ketimines to accomplish the direct asymmetric aza-vinylogous-type Mannich reactions. A series of densely functionalized nitrones with vicinal tertiary-quaternary stereogenic centers are furnished in high yields (70%~97%) with good to excellent stereoselectivity (83%~99% ee, >19:1 dr). Moreover, subsequent[3+2] dipolar cycloaddition reactions between the chiral nitrones and activated alkenes can be realized in exclusive diastereoselectivity, producing complex spirocyclic indolenine architectures incorporating a hydrogenated isoxazole ring. These nitrones, as a new type of aza-vinylogous nucleophiles, may have a wide range of applications in asymmetric synthesis in the future. A representative procedure for the asymmetric aza-vinylogous-type Mannich reaction is as follows:nitrone 1 (0.1 mmol), ketimine 2 (0.11 mmol), catalyst C5 (0.01 or 0.02 mmol) are added into an oven-dried vial equipped with a magnetic stirbar. Xylene (1.0 mL) is added and the mixture is stirred at 50℃ and monitored by TLC. After completion, the residue is purified by flash column chromatography on silica gel eluting with petroleum ether/ethyl acetate (15:1 to 5:1) to afford the product 3.

[1] (a) Schneider, C.; Sickert, M. In Chiral Amine Synthesis, Ed.:Nugent, T. C., Wiley-VCH, Weinheim, 2010.
(b) Roselló, M. S.; del Pozo, C.; Fustero, S. Synthesis 2016, 48, 2553.
(c) Martin, S. F. Acc. Chem. Res. 2002, 35, 895.
[2] (a) Yamaguchi, A.; Matsunaga, S.; Shibasaki, M. Org. Lett. 2008, 10, 2319.
(b) Shepherd, N. E.; Tanabe, H.; Xu, Y.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2010, 132, 3666.
(c) Zhou, L.; Lin, L.; Ji, J.; Xie, M.; Liu, X.; Feng, X. Org. Lett. 2011, 13, 3056.
(d) Guo, Y.-L.; Bai, J.-F.; Peng, L.; Wang, L.-L.; Jia, L.-N.; Luo, X.-Y.; Tian, F.; Xu, X.-Y.; Wang, L.-X. J. Org. Chem. 2012, 77, 8338.
(e) Yin, L.; Takada, H.; Kumagai, N.; Shibasaki, M. Angew. Chem. Int. Ed. 2013, 125, 7451.
(f) Guo, Y.; Zhang, Y.; Qi, L.; Tian, F.; Wang, L. RSC Adv. 2014, 4, 27286.
(g) Nakamura, S.; Yamaji, R.; Hayashi, M. Chem. Eur. J. 2015, 21, 9615.
[3] (a) Liu, T.-Y.; Cui, H.-L.; Long, J.; Li, B.-J.; Wu, Y.; Ding, L.-S.; Chen, Y.-C. J. Am. Chem. Soc. 2007, 129, 1878.
(b) Niess, B.; J?rgensen, K. A. Chem. Commun. 2007, 1620.
(c) Chen, Q.-A.; Zeng, W.; Wang, D.-W.; Zhou, Y.-G. Synlett 2009, 2009, 2236.
(d) Xiong, X.-F.; Jia, Z.-J.; Du, W.; Jiang, K.; Liu, T.-Y.; Chen, Y.-C. Chem. Commun. 2009, 6994.
(e) Cheng, C.; Lu, X.; Ge, L.; Chen, J.; Cao, W.; Wu, X.; Zhao, G. Org. Chem. Front. 2017, 4, 101.
[4] Zhang, H.-J.; Shi, C.-Y.; Zhong, F.; Yin, L. J. Am. Chem. Soc. 2017, 139, 2196.
[5] (a) Curti, C.; Rassu, G.; Zambrano, V.; Pinna, L.; Pelosi, G.; Sartori, A.; Battistini, L.; Zanardi, F.; Casiraghi, G. Angew. Chem. Int. Ed. 2012, 51, 6200.
(b) Rassu, G.; Zambrano, V.; Pinna, L.; Curti, C.; Battistini, L.; Sartori, A.; Pelosi, G.; Zanardi, F.; Casiraghi, G. Adv. Synth. Catal. 2013, 355, 1881.
(c) Chen, Q.; Wang, G.; Jiang, X.; Xu, Z.; Lin, L.; Wang, R. Org. Lett. 2014, 16, 1394.
(d) Ranieri, B.; Sartori, A.; Curti, C.; Battistini, L.; Rassu, G.; Pelosi, G.; Casiraghi, G.; Zanardi, F. Org. Lett. 2014, 16, 932.
(e) Di Iorio, N.; Righi, P.; Ranieri, S.; Mazzanti, A.; Margutta, R. G.; Bencivenni, G. J. Org. Chem. 2015, 80, 7158.
(f) Xiao, X.; Mei, H.; Chen, Q.; Zhao, X.; Lin, L.; Liu, X.; Feng, X. Chem. Commun. 2015, 51, 580.
(g) Zheng, C.; Chen, W.-X.; Chen, F.-E. Asian J. Org. Chem. 2015, 4, 1044.
(h) Han, J.-L.; Chang, C.-H. Chem. Commun. 2016, 52, 2322.
(i) Tian, L.; Hu, X.-Q.; Li, Y.-H.; Xu, P.-F. Chem. Commun. 2013, 49, 7213.
(j) Sun, Q.; Li, X.; Su, J.; Zhao, L.; Ma, M.; Zhu, Y.; Zhao, Y.; Zhu, R.; Yan, W.; Wang, K.; Wang, R. Adv. Synth. Catal. 2015, 357, 3187.
(k) Li, X.; Su, J.; Liu, Z.; Zhu, Y.; Dong, Z.; Qiu, S.; Wang, J.; Lin, L.; Shen, Z.; Yan, W.; Wang, K.; Wang, R. Org. Lett. 2016, 18, 956.
[6] Liu, Y.; Yang, Y.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Synlett 2015, 26, 67.
[7] (a) Chen, Y.-R.; Zhan, G.; Du, W.; Chen, Y.-C. Adv. Synth. Catal. 2016, 358, 3759. For other examples with nitrone ylides, see:
(b) Hanessian, S.; Bayrakdarian, M. Tetrahedron Lett. 2002, 43, 967.
(c) Hanessian, S.; Bayrakdarian, M. Tetrahedron Lett. 2002, 43, 9441.
(d) Merino, P.; Tejero, T.; Díez-Martínez, A.; Gültekin, Z. Eur. J. Org. Chem. 2011, 2011, 6567.
(e) Merino, P.; Tejero, T.; Díez-Martínez, A. J. Org. Chem. 2014, 79, 2189.
(f) Juste-Navarro, V.; Delso, L.; Tejero, T.; Merino, P. Chem. Eur. J. 2016, 22, 11527.
(g) Prieto, L.; Juste-Navarro, V.; Uria, U.; Delso, I.; Reyes, E.; Tejero, T.; Carrillo, L.; Merino, P.; Vicario, J. L. Chem. Eur. J. 2017, 23, 2764.
[8] Zhan, G.; Shi, M.-L.; Lin, W.-J.; Ouyang, Q.; Du, W.; Chen, Y.-C. Chem. Eur. J. 2017, 23, 6286.
[9] For selected examples, see:(a) Feng, J.; Yan, W.; Wang, D.; Li, P.; Sun, Q.; Wang, R. Chem. Commun. 2012, 48, 8003.
(b) Hara, N.; Nakamura, S.; Sano, M.; Tamura, R.; Funahashi, Y.; Shibata, N. Chem. Eur. J. 2012, 18, 9276.
(c) Yan, W.; Wang, D.; Feng, J.; Li, P.; Zhao, D.; Wang, R. Org. Lett. 2012, 14, 2512.
(d) Zhao, J.; Fang, B.; Luo, W.; Hao, X.; Liu, X.; Lin, L.; Feng, X. Angew. Chem. Int. Ed. 2015, 54, 241.
(e) Wang, Y.; Cao, Z.; Niu, Y.; Zhao, X.; Zhou, J. Acta Chim. Sinica 2014, 72, 867. (王雨卉, 曹中艳, 牛艳霏, 赵小莉, 周剑, 化学学报, 2014, 72, 867.)
(f) Liu, Y.-L.; Zhou, J. Chem. Commun. 2013, 49, 4421.
(g) Yin, X.; Xue, P.; Dong, K.; Liao, K.; Zhou, F.; Zhou, J. Acta Chim. Sinica 2015, 73, 685. (尹小平, 徐鹏巍, 董坤, 廖奎, 周锋, 周剑, 化学学报, 2015, 73, 685.)
[10] (a) Li, B.-J.; Jiang, L.; Liu, M.; Chen, Y.-C.; Ding, L.-S.; Wu, Y. Synlett 2005, 603.
(b) Connon, S. J. Chem. Commun. 2008, 2499.
(c) Takemoto, Y. Chem. Pharm. Bull. 2010, 58, 593.
(d) Taylor, M. S.; Jacobsen, E. N. Angew. Chem. Int. Ed. 2006, 45, 1520.
[11] CCDC-1558319(4) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www. ccdc. cam. ac. uk/data_request/cif.
/
| 〈 |
|
〉 |