

应用纳米零价铁富集银的研究
收稿日期: 2017-07-28
网络出版日期: 2017-09-18
基金资助
“博新计划”(BX201700172)和国家自然科学基金(No.51578398)资助.
Enrichment of Silver from Water Using Nanoscale Zero-Valent Iron (nZVI)
Received date: 2017-07-28
Online published: 2017-09-18
Supported by
Project supported by the National Postdoctoral Program for Innovative Talents (BX201700172) and the National Natural Science Foundation of China (No. 51578398).
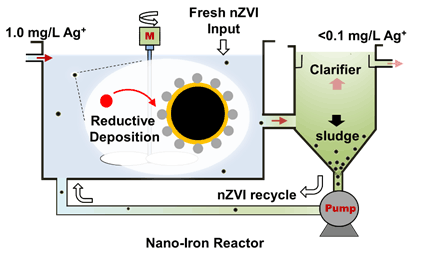
大量研究表明,纳米零价铁(nanoscale Zero-Valent Iron,nZVI)对水中重金属,尤其是金、银等稀贵金属,有良好的分离富集作用.利用纳米零价铁反应器证明了nZVI可从废水中分离低浓度的银离子(Ag+),并生成高含量的“银矿石”.此外,也证明了反应区氧化还原电位能够反映nZVI与Ag+的反应速率和分离效率.利用X射线衍射仪、X射线光电子能谱和高分辨透射电子显微镜等手段对反应产物进行表征,证实了Ag+可被nZVI还原为单质银,并以纳米颗粒的形式(<10 nm)沉积在nZVI表面.与其他材料(常见吸附/还原材料)相比,nZVI具有效率高,受pH影响小的优点.研究结果表明,nZVI是一种能够高效富集痕量银资源并产生高价值纳米银的材料.
顾天航 , 石君明 , 滑熠龙 , 刘静 , 王伟 , 张伟贤 . 应用纳米零价铁富集银的研究[J]. 化学学报, 2017 , 75(10) : 991 -997 . DOI: 10.6023/A17070345
Increasing evidence suggests that nanoscale zero-valent iron (nZVI) is an effective nanomaterial for the enrichment and separation of heavy metals from water, especially for recovering precious metals such as gold and silver from trace level sources. In this work, a nano-iron reactor, consisting of reaction zone, separation zone and reuse facilities, is applied to recovery of silver from aqueous solution using nZVI. We demonstrate that nZVI could sequester Ag+ (ca. 1 mg/L) and be transformed into high-grade (32.0 mg/g) silver solids ("ore") as nZVI is recycled in this "reaction-separation-reuse" system. Besides, increasing hydraulic retention time (HRT), from 10 min to 60 min, could enhance the enrichment efficiency and finally improve silver content in solid phase. We further demonstrate that there is a positive correlation between solution oxidation-reduction potential in reaction zone and Ag+ concentration in effluent, and this relationship can be used to regulate the reaction kinetics and separation efficiency. Data from oxidation-reduction potential regulating experiment are presented and a mathematic formula is provided, proving this system is reliable and controllable. Solid phase characterizations with X-ray diffraction and X-ray photoelectron spectroscopy confirm that Ag+ is reduced to metallic silver (Ag0). Images acquired via high-resolution transmission electron microscopy reveal that Ag0 (<10 nm) is deposited on the surface of nZVI (Ag-nZVI). Pure silver nanoparticles (AgNPs, 9~32 nm) could be acquired by simply processing Ag-nZVI with sulfuric acid and polyvinyl pyrrolidone. Batch experiments confirm that nZVI is far more efficient and less pH-dependent, comparing to other materials (e.g., mZVI, α-Fe2O3, nTiO2). 99% Ag+ (1000 mg/L) could be sequestrated in less than 15 s with 1 g/L nZVI. And the separation coefficient of nZVI for Ag+ reaches 3.2×104, which is several orders of magnitude higher than that of conventional adsorbents and reductants (102~741). This study demonstrates that nZVI is a powerful candidate to recover Ag from water (e.g., industrial wastewater, groundwater) with trace level silver and produce valuable AgNPs.

[1] Yu, S. L.; Yin, Y. G.; Liu, J. F. Environ. Sci.-Proc. Imp. 2013, 15, 78.
[2] Syed, S. Waste. Manage. 2016, 50, 234.
[3] World Silver Survey, 2017, GFMS Limited/The Silver Institute. http://www.silverinstitute.org
[4] Benn, M. T.; Westerhoff, P. Environ. Sci. Technol. 2008, 42, 4133.
[5] Zhou, X. X.; Liu, J. F.; Yuan, C. G.; Chen, Y. S. J. Anal. Atom. Spectrom. 2016, 31, 2285.
[6] Eckelman, M. J.; Graedel, T. E. Environ. Sci. Technol. 2007, 41, 6283.
[7] Li, R.; Lu, Y. Y.; Lei, K. X.; Li, F. J.; Cheng, F. Y.; Chen, J. Acta Chim. Sinica 2017, 75, 199(in Chinese). (李冉, 卢艳莹, 雷凯翔, 李福军, 程方益, 陈军, 化学学报, 2017, 75, 199.)
[8] Wang, C.; Deng, N.; Wang, L. L.; Xu, D. J.; Yao, X. Q. Chinese J. Org. Chem. 2016, 36, 1034(in Chinese). (王超, 邓楠, 王玲玲, 许定健, 姚小泉, 有机化学, 2016, 36, 1034.)
[9] Vance, M. E.; Kuiken, T.; Vejerano, E. P.; McGinnis, S. P.; Hochella, M. F.; Rejeski, D.; Hull, M. S. Beilstein. J. Nanotech. 2015, 6, 1769.
[10] Song, X. H.; Gunawan, P.; Jiang, R. R.; Leong, S. S. J.; Wang, K.; Xu, R. J. Hazard. Mater. 2011, 194, 162.
[11] Zhou, Y. M.; Gao, B.; Zimmerman, R. A.; Cao, X. D. Chemosphere 2014, 117, 801.
[12] Celik. Z.; Gulfen. M.; Aydin, A. O. J. Hazard. Mater. 2010, 174, 556.
[13] Wang, H. Y.; Gao, H.; Sun, J. S.; Li, J.; Su, Y. X.; Ji, Y. L.; Gong, C. M. Desalination 2011, 270, 258.
[14] Huo, H. Y.; Su, H. J.; Tan, T. W. Chem. Eng. J. 2009, 150, 139.
[15] Huang, X. Y.; Wang, W.; Ling, L.; Zhang, W. X. Acta Chim. Sinica 2017, 75, 529(in Chinese). (黄潇月, 王伟, 凌岚, 张伟贤, 化学学报, 2017, 75, 529.)
[16] Mu, Y.; Jia, F. L.; Ai, Z. H.; Zhang, L. Z. Environ. Sci.-Nano 2017, 4, 27.
[17] Fu, F. L.; Dionysiou, D. D.; Liu, H. J. Hazard. Mater. 2014, 267, 194.
[18] Zhang, Y. L.; Yan, J.; Dai, C. M.; Li, Y. T.; Zhou, Y.; Zhou, X. F. J. Nanopart. Res. 2015, 17, 1110.
[19] Teng, W.; Fan, J. W.; Wang, W.; Bai, N.; Liu, R.; Liu, Y.; Deng, Y. H.; Kong, B.; Yang, J. P.; Zhao, D. Y.; Zhang, W. X. J. Mater. Chem. A 2017, 5, 4478.
[20] Ling, L.; Zhang, W. X. J. Am. Chem. Soc. 2015, 137, 2788.
[21] Sheng, G. D.; Yang, P. J.; Tang, Y. N.; Hu, Q. Y.; Li, H.; Ren, X. M.; Hu, B. W.; Wang, X. K.; Huang, Y. Y. Appl. Catal. B-Environ. 2016, 193, 189.
[22] Xia, X. F.; Hua, Y. L.; Huang, X. Y.; Ling, L.; Zhang, W. X. Acta Chim. Sinica 2017, 75, 594(in Chinese). (夏雪芬, 滑熠龙, 黄潇月, 凌岚, 张伟贤, 化学学报, 2017, 75, 594.)
[23] Sheng, G. D.; Alsaedi, A.; Shammakh, W.; Monaquel, S.; Sheng, J.; Wang, X. K.; Li, H.; Huang, Y. Y. Appl. Carbon. 2016, 99, 123.
[24] Li, S. L.; Wang, W.; Liu, Y. Y.; Zhang, W. X. Chem. Eng. J. 2014, 254, 115.
[25] Wang, W.; Hua, Y. L.; Li, S. L.; Yan, W. L.; Zhang, W. X. Chem. Eng. J. 2016, 304, 79.
[26] Li, S. L.; Wang, W.; Liang, F. P.; Zhang, W. X. J. Hazard. Mater. 2017, 322, 163.
[27] Wang, W.; Li, S. L.; Lei, H.; Pan, B. C.; Zhang, W. X. Chem. Eng. J. 2015, 260, 616.
[28] Shi, Z. Q.; Nurmi, T. J.; Tratnyek, G. P. Environ. Sci. Technol. 2011, 45, 1586.
[29] Sverdrup, H.; Koca, D.; Ragnarsdottir, V. K. Resour. Conserv. Recy. 2014, 83, 121.
[30] Liang, L. P.; Yang, W. J.; Guan, X. H.; Li, J. L.; Xu, Z. J.; Wu, J.; Huang, Y. Y.; Zhang, X. Z. Water Res. 2013, 47, 5846.
[31] Guan, X. H.; Sun, Y. K.; Qin, H. J.; Li, J. X.; Lo, I. M. C.; He, D.; Dong, H. R. Water Res. 2015, 75, 224.
[32] Liang, L. P.; Sun, W.; Guan, X. H.; Huang, Y. Y.; Choi, W. Y.; Bao, H. L.; Li, L. N.; Jiang, Z. Water Res. 2014, 49, 371.
[33] Nitayaphat, W.; Jintakosol, T. J. Clean. Prod. 2015, 87, 850.
[34] Wang, Y.; Ma, X. J.; Li, Y. F.; Li, X. L.; Yang, L. Q.; Ji, L.; He, Y. Chem. Eng. J. 2012, 209, 394.
[35] Ju, S. H.; Zhang, Y. F.; Zhang, Y.; Xue, P. Y.; Wang, Y. H. J. Hazard. Mater. 2011, 192, 554.
[36] Yin, Y. G.; Shen, M. H.; Tan, Z. Q.; Yu, S. J.; Liu, J. F.; Jiang, G. B. Environ. Sci. Technol. 2015, 49, 6581.
/
| 〈 |
|
〉 |