

钯配合物和手性磷酸连续催化的烯丙醇和醛的不对称羰基烯丙基化反应
收稿日期: 2017-08-14
网络出版日期: 2017-10-09
基金资助
项目受国家自然科学基金(No.21232007)资助.
Asymmetric Carbonyl Allylation of Aldehydes with Allylic Alcohols under the Sequential Catalysis of Palladium Complex and Chiral Phosphoric Acid
Received date: 2017-08-14
Online published: 2017-10-09
Supported by
Project supported by the National Natural Science Foundation of China (No. 21232007).
张子競, 陶忠林, 阿拉法特·阿地力, 龚流柱 . 钯配合物和手性磷酸连续催化的烯丙醇和醛的不对称羰基烯丙基化反应[J]. 化学学报, 2017 , 75(12) : 1196 -1201 . DOI: 10.6023/A17080372
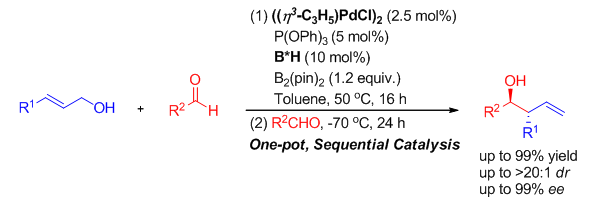
The asymmetric carbonyl allylation of aldehydes with allylmetal reagents presents one of the most efficient and straightforward methods for the synthesis of optically active homoallylic alcohols, which have found widespread applications in organic synthesis. As such, a wide range of chiral catalysts, including Lewis acids, Lewis bases and Brønsted acids have been reported to enable highly stereoselective carbonyl allylation of aldehydes with allylmetal reagents. Among them, chiral phosphoric acid-catalyzed carbonyl allylation of aldehydes with pinacol allylboronates represents a promising method, whereas an additional operations required for the preparation of allylboronates from allyl halides or highly active allylmetallics impose some constraints on the carbonyl allylation process. In this context, the asymmetric addition of allylboronates, in situ generated from palladium-catalyzed allylborylation, to aldehydes has been reported, while stoichiometric amounts of chiral diboronate reagents are basically required. Allylic alcohols are readily available feedstock. The direct use of allylic alcohols as starting materials in asymmetric allylborylation of carbonyls is highly valuable. Herein, we will report an asymmetric carbonyl allylation of aldehydes with allylic alcohols in the presence of octamethyl-2,2'-bi(1,3,2-dioxaborolane) under the sequential catalysis of a palladium complex and chiral phosphoric acid. The presence of 2.5 mol% (η3-C3H5)2Pd2Cl2, 5 mol% P(OPh)3 and 10 mol% chiral phosphoric acid B*H-1 enabled 4-nitrobenzaldehyde 2a to smoothly undergo the asymmetric carbonyl allylation reaction with 2-buten-1-ol 1a and octamethyl-2,2'-bi(1,3,2-dioxaborolane), giving rise to the desired homoallylic alcohol product 3aa in a 99% yield and with >20:1 dr and 92% ee. Under the optimal conditions, the generality for allylic alcohol substrates was investigated to reveal that the installation of either of saturated alkyl substituents, carbon-carbon double bond or heteroatom group in the allylic alcohols allowed the target products (3ca~3fa, 3ha~3ja) to be obtained in high yields and with excellent stereoselectivities. A (Z)-allylic alcohol and branched allylic alcohols were also able to generate the target products (3ba, 3ga), successfully. Although cinnamic alcohols participated in a clean reaction, relatively lower yields and stereoselectivity were delivered (3ka and 3la). The examination of aldehydes suggested that the introduction of either electronically deficient or rich substituents to the benzene ring of benzaldehydes was tolerant and led to corresponding homoallylic alcohols in excellent yields and stereoselectivities (3ab~3ak and 3m), with the exception of o-anisaldehyde (3al). In addition, 2-naphthaldehyde, aliphatic aldehydes and enals are all good substrates and provide high yields and enantiomeric excesses as exemplified by 3-phenylpropanal and 4-methoxycinnamaldehyde (3an~3ap).
[1] (a) Chemler, S. R.; Roush, W. R. In Modern Carbonyl Chemistry, Ed.: Otera, J., Wiley-VCH, Weinheim, Germany, 2000, pp. 403~490;
(b) Elford, T. G.; Hall, D. G. Synthesis 2010, 893;
(c) Yus, M.; González-Gómez, J. C.; Foubelo, F. Chem. Rev. 2013, 113, 5595.
[2]
(a) Yamamoto, Y.; Asao, N. Chem. Rev. 1993, 93, 2207;
(b) Denmark, S. E.; Fu, J. Chem. Rev. 2003, 103, 2763;
(c) Yus, M.; González-Gómez, J. C.; Foubelo, F. Chem. Rev. 2011, 111, 7774.
[3] For examples with chiral Lewis acids as catalysts, see:
(a) Furuta, K.; Mouri, M.; Yamamoto, H. Synlett 1991, 561;
(b) Costa, A. L.; Piazza, M. G.; Tagliavini, E.; Trombini, C.; Umani-Ronchi, A. J. Am. Chem. Soc. 1993, 115, 7001;
(c) Keck, G. E.; Tarbet, K. H.; Geraci, L. S. J. Am. Chem. Soc. 1993, 115, 8467;
(d) Ishiyama, T.; Ahiko, T.-A.; Miyaura, N. J. Am. Chem. Soc. 2002, 124, 12414;
(e) Wada, R.; Oisaki, K.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2004, 126, 8910.
[4] For examples with chiral Lewis bases as catalysts, see:
(a) Denmark, S. E.; Fu, J. J. Am. Chem. Soc. 2001, 123, 9488;
(b) Malkov, A.; Orsini, M.; Pernazza, D.; Muir, K. W.; Langer, V.; Meghani, P.; Kocovsky, P. Org. Lett. 2002, 4, 1047;
(c) Denmark, S. E.; Fu, J. J. Am. Chem. Soc. 2003, 125, 2208;
(d) Malkov, A. V.; Bell, M.; Castelluzzo, F.; Kocovsky, P. Org. Lett. 2005, 7, 3219;
(e) Denmark, S. E.; Fu, J.; Coe, D. M.; Su, X.; Pratt, N. E.; Griedel, B. D. J. Org. Chem. 2006, 71, 1513;
(f) Malkov, A. V.; Ramirez-Lopez, P.; Biedermannova, L.; Rulisek, L.; Dufkova, L.; Kotora, M.; Zhu, F.; Kocovsky, P. J. Am. Chem. Soc. 2008, 130, 5341;
(g) Bai, B.; Yang, J.; Zhang, G.-H.; Mao, D.-B. Chin. J. Org. Chem. 2015, 35, 975(in Chinese). (白冰, 杨静, 张改红, 毛多斌, 有机化学, 2015, 35, 975.)
[5] For examples with chiral Brønsted acids as catalysts, see:
(a) Rauniyar, V.; Hall, D. G. Angew. Chem., Int. Ed. 2006, 45, 2426;
(b) Rauniyar, V.; Zhai, H.; Hall, D. G. J. Am. Chem. Soc. 2008, 130, 8481;
(c) Rauniyar, V.; Hall, D. G. J. Org. Chem. 2009, 74, 4236;
(d) Jain, P.; Antilla, J. C. J. Am. Chem. Soc. 2010, 132, 11884;
(e) Xing, C.-H.; Liao, Y.-X.; Zhang, Y.; Sabarova, D.; Bassous, M.; Hu, Q.-S. Eur. J. Org. Chem. 2012, 1115.
[6] For other examples of asymmetric carbonyl allylation reactions, see:
(a) Kim, I. S.; Ngai, M.; Krische, M. J. J. Am. Chem. Soc. 2008, 130, 14891;
(b) Lou, S.; Moquist, P. N.; Schaus, S. E. J. Am. Chem. Soc. 2006, 128, 12660;
(c) Barnett, D. S.; Moquist, P. N.; Schaus, S. E. Angew. Chem., Int. Ed. 2009, 48, 8679.
[7] For the selected achiral examples, see:
(a) Sebelius, S.; Wallner, O. A.; Szabó, K. J. Org. Lett. 2003, 5, 3065;
(b) Selander, N.; Sebelius, S.; Estay, C.; Szabó, K. J. Eur. J. Org. Chem. 2006, 4085;
(c) Se- lander, N.; Kipke, A.; Sebelius, S.; Szabó, K. J. J. Am. Chem. Soc. 2007, 129, 13723;
(d) Olsson, V. J.; Szabó, K. J. Angew. Chem., Int. Ed. 2007, 46, 6891;
(e) Selander, N.; Szabó, K. J. Chem. Commun. 2008, 3420;
(f) Zhou, Y.-H.; Wang, H.; Liu, Y.; Zhao, Y.-L.; Zhang, C.-X.; Qu, J.-P. Org. Chem. Front. 2017, 4, 1580.
[8] For the examples of using chiral diboronates, see:
(a) Sebelius, S.; Szabó, K. J. Eur. J. Org. Chem. 2005, 2539;
(b) Vogt, M.; Ceylan, S.; Kirschning, A. Tetrahedron 2010, 66, 6450.
[9]
(a) Zanoni, G.; Gladiali, S.; Marchetti, A.; Piccinini, P.; Tredici, I.; Vidari, G. Angew. Chem., Int. Ed. 2004, 43, 846;
(b) Zhu, S.-F.; Yang, Y.; Wang, L.-X.; Liu, B.; Zhou, Q.-L. Org. Lett. 2005, 7, 2333;
(c) Howell, G. P.; Minnaard, A. J.; Feringa, B. L. Org. Biomol. Chem. 2006, 4, 1278;
(d) Yu, Y.-N.; Xu, M.-H. Acta Chim. Sinica 2017, 75, 655(in Chinese). (于月娜, 徐明华, 化学学报, 2017, 75, 655.)
[10]
(a) Zhu, S.-F.; Qiao, X.-C.; Zhang, Y.-Z.; Wang, L.-X.; Zhou, Q.-L. Chem. Sci. 2011, 2, 1135;
(b) Tsukamoto, H.; Kawase, A.; Doi, T. Chem. Commun. 2015, 51, 8027.
[11] Yatagai, M.; Yamagishi, T.; Hida, M. Bull. Chem. Soc. Jpn. 1984, 57, 823.
(b) McIntosh, J. M.; Leavitt, R. K. Tetrahedron Lett. 1986, 27, 3839.
(c) Jiang, Y. Z.; Liu, G.; Zhou, C. Y.; Piao, H. R.; Wu, L. J.; Mi, A. Q. Synth. Commun. 1991, 21, 1087.
[12]
(a) Li, L.-L.; Tao, Z.-L.; Han, Z.-Y.; Gong, L.-Z. Org. Lett. 2017, 19, 102;
(b) Tang, H.-M.; Huo, X.-H.; Meng, Q.-H.; Zhang, W.-B. Acta Chim. Sinica 2016, 74, 219(in Chinese). (汤淏溟, 霍小红, 孟庆华, 张万斌, 化学学报, 2016, 74, 219.)
[13]
(a) Grayson, M. N.; Pellegrinet, S. C.; Goodman, J. M. J. Am. Chem. Soc. 2012, 134, 2716.
(b) Wang, H.; Jain, P.; Antilla, J. C.; Houk, K. N. J. Org. Chem. 2013, 78, 1208.
(c) Incerti-Pradillos, C. A.; Kabeshov, M. A.; Malkov, A. V. Angew. Chem., Int. Ed. 2013, 52, 5338.
[14]
(a) Chen, G.; Deng, Y.; Gong, L.; Mi, A.; Cui, X.; Jiang, Y.; Choi, M. C. K.; Chan, A. S. C. Tetrahedron: Asymmetry 2001, 12, 1567.
(b) Nakoji, M.; Kanayama, T.; Okino, T.; Takemoto, Y. Org. Lett. 2001, 3, 3329.
(c) Park, Y. J.; Park, J.-W.; Jun, C.-H. Acc. Chem. Res. 2008, 41, 222.
(d) Shao, Z.; Zhang, H. Chem. Soc. Rev. 2009, 38, 2745.
(e) Du, Z.; Shao, Z. Chem. Soc. Rev. 2013, 42, 1337.
(f) Wu, X.; Li, M.-L.; Gong, L.-Z. Acta Chim. Sinica 2013, 71, 1091(in Chinese). (吴祥, 李明丽, 龚流柱, 化学学报, 2013, 71, 1091.)
(g) Chen, D.-F.; Han, Z.-Y.; Zhou, X.-L.; Gong, L.-Z. Acc. Chem. Res. 2014, 47, 2365.
(h) Yang, Z.-P.; Zhang, W.; You, S.-L. J. Org. Chem. 2014, 79, 7785.
[15] Jiang, G.-X.; List, B. Angew. Chem., Int. Ed. 2011, 50, 9471.
[16] Tao, Z.-L.; Zhang, W.-Q.; Chen, D.-F.; Arafate Adele; Gong, L.-Z. J. Am. Chem. Soc. 2013, 135, 9255.
/
| 〈 |
|
〉 |