

自掺杂氮多孔交联碳纳米片在超级电容器中的应用
收稿日期: 2017-09-17
网络出版日期: 2017-12-13
基金资助
项目受国家自然科学基金(Nos.51572052,21503055)资助.
Self N-Doped Porous Interconnected Carbon Nanosheets Material for Supercapacitors
Received date: 2017-09-17
Online published: 2017-12-13
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 51572052, 21503055).
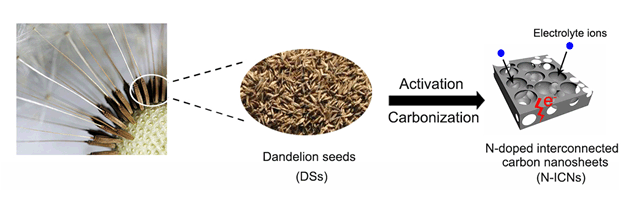
自掺杂氮的多孔交联碳纳米片(N-ICNs)是将蒲公英种子通过一步活化碳化法制备的.蒲公英种子本身富含氮,不需要进行额外的掺杂处理,可以作为理想的碳前驱体.通过X射线衍射(XRD)、扫描电子显微镜(SEM)、透射电子显微镜(TEM)对所制备的碳材料的微观形貌和组成成分进行了表征.基于高含氮量(2.88%),N-ICNs在1 A·g-1下具有337 F·g-1的比电容和优异的倍率性能.此外,由N-ICNs组合成的对称型超级电容器在操作电压范围为0~2 V时具有很高的能量密度(25.3 Wh·kg-1)和功率密度(900 W·kg-1),并且在循环10000次后仍具有98%的电容保持率.因此,N-ICNs将是一种非常理想的电极材料.
赵婧 , 龚俊伟 , 李一举 , 程魁 , 叶克 , 朱凯 , 闫俊 , 曹殿学 , 王贵领 . 自掺杂氮多孔交联碳纳米片在超级电容器中的应用[J]. 化学学报, 2018 , 76(2) : 107 -112 . DOI: 10.6023/A17090422
Self N-doped porous cross-linked carbon nanosheets (N-ICNs) are prepared by one-step activation carbonization using dandelion seeds. The dandelion seeds are rich in nitrogen without any additional doping treatment, which can be served as an ideal carbon precursor. The microstructure and composition of the prepared carbon materials are characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). It can be seen from the SEM and TEM spectra that the N-ICNs exhibit the porous interconnected structure, which can facilitate the transfer of the electrons and the dispersion of the electrolyte ions. Moreover, the XRD spectra show the defects in the amorphous carbon material. Nitrogen adsorption/desorption isotherms of the N-ICNs show a high specific surface area of 1564 m2·g-1, and the pore size distribution shows numerous micropores and macropores, which contributes to the formation of double layer capacitance and the accessibility of the electrolyte ions. The wide-scan spectra present the presence of C, N and O atoms. Interestingly, the N content of the N-ICNs without any extra doping treatment is high (2.88%). Based on the high nitrogen content, the N-ICNs exhibit a good specific capacitance of 337 F·g-1 at a current density of 1 A·g-1 with an excellent capacitance retention of 99% after 10000 cycles. The good electrochemical performances mainly caused by the nitrogen functional groups in the carbon lattice, which can improve the wettability as well as provide pseudocapacitance due to the redox reactions of amine groups. In addition, the symmetric supercapacitor assembled with N-ICNs in the operating voltage range of 0~2 V shows high energy density of 25.3 Wh·kg-1 at the power density of 900 W·kg-1, which are superior than the other carbon materials reported. And the capacitance retention can retain 98% after 10000 cycles. Therefore, the low-cost biomass-derived porous interconnected carbon material can be a promising electrode material for supercapacitors.

[1] Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Adv. Energy Mater. 2014, 4, 157.
[2] Wu, Z.; Li, L.; Yan, J.; Zhang, X. Adv. Sci. 2017, 4, 1600382.
[3] Li, T.; Zhao, J.; Li, Y.; Quan, Z.; Xu, J. Acta Chim. Sinica 2017, 75, 485. (李甜甜, 赵继宽, 李尧, 全贞兰, 徐洁, 化学学报, 2017, 75, 485.)
[4] Jin, Y.; Chen, H.; Chen, M.; Liu, N.; Li, Q. ACS Appl. Mater. Interfaces 2013, 5, 3408.
[5] Su, S.; Lai, Q.; Liang, Y. Acta Chim. Sinica 2015, 73, 735. (苏善金, 来庆学, 梁彦瑜, 化学学报, 2015, 73, 735.)
[6] Hsu, Y. H.; Lai, C. C.; Ho, C. L.; Lo, C. T. Electrochim. Acta 2014, 127, 369.
[7] Davies, A.; Audette, P.; Farrow, B.; Hassan, F.; Chen, Z.; Yu, A. J. Phys. Chem. C 2011, 115, 17612.
[8] Li, Z.; Zhang, L.; Amirkhiz, B. S.; Tan, X.; Xu, Z.; Wang, H.; Olsen, B. C.; Holt, C. M. B.; Mitlin, D. Adv. Energy Mater. 2012, 2, 431.
[9] Chen, W.; Zhang, H.; Huang, Y.; Wang, W. J. Mater. Chem. 2010, 20, 4773.
[10] Liu, D.; Yu, S.; Shen, Y.; Chen, H.; Shen, Z.; Zhao, S.; Fu, S.; Yu, Y.; Bao, B. Ind. Eng. Chem. Res. 2015, 54, 12570.
[11] Hu, Z.; Li, S.; Cheng, P.; Yu, W.; Li, R.; Shao, X.; Lin, W.; Yuan, D. J. Mater. Sci. 2016, 51, 2627.
[12] Dou, S.; Huang, X.; Ma, Z.; Wu, J.; Wang, S. Nanotechnology 2015, 26, 045402.
[13] Wang, C.; Qiu, F.; Deng, H.; Zhang, X.; He, P.; Zhou, H. Acta Chim. Sinica 2017, 75, 241. (王超强, 邱飞龙, 邓瀚, 张晓禹, 何平, 周豪慎, 化学学报, 2017, 75, 241.)
[14] Wan, G.; Fu, Y.; Guo, J.; Xiang, Z. Acta Chim. Sinica 2015, 73, 557. (万刚, 付宇昂, 郭佳宁, 向中华, 化学学报, 2015, 73, 557.)
[15] Dias, A.; Ciminelli, V. S. T. Ferroelectrics 2000, 241, 9.
[16] Xu, J.; He, F.; Gai, S.; Zhang, S.; Li, L.; Yang, P. Nanoscale 2014, 6, 10887.
[17] Bello, A.; Manyala, N.; Barzegar, F.; Khaleed, A. A.; Momodu, D. Y.; Dangbegnon, J. K. RSC Adv. 2016, 6, 1800.
[18] Liu, B.; Zhou, X.; Chen, H.; Liu, Y.; Li, H. Electrochim. Acta 2016, 208, 55.
[19] Rufford, T. E.; Hulicova-Jurcakova, D.; Zhu, Z.; Lu, G. Q.; Electrochem. Commun. 2008, 10, 1594.
[20] Zhong, Y.; Xia, X.; Deng, S.; Zhan, J.; Fang, R.; Xia, Y.; Wang, X.; Zhang, Q.; Tu, J. Adv. Energy Mater. 2017, 201701110.
[21] Cao, H.; Zhou, X.; Qin, Z.; Liu, Z. Carbon 2013, 56, 218.
[22] Yang, J.; Jo, M. R.; Kang, M.; Huh, Y. S.; Jung, H.; Kang, Y.-M. Carbon 2014, 73, 106.
[23] Zhao, L.; Fan, L. Z.; Zhou, M. Q.; Guan, H.; Qiao, S.; Antonietti, M.; Titirici, M. M. Adv. Mater. 2010, 22, 5202.
[24] Long, C.; Chen, X.; Jiang, L.; Zhi, L.; Fan, Z. Nano Energy 2015, 12, 141.
[25] Jiang, L.; Sheng, L.; Long, C.; Fan, Z. Nano Energy 2015, 11, 471.
[26] Xu, X.; Wang, M.; Liu, Y.; Li, Y.; Lu, T.; Pan, L. Energy Storage Mater. 2016, 5, 132.
[27] Raymundo-Pinero, E.; Cadek, M.; Beguin, F. Adv. Funct. Mater. 2009, 19, 1032.
[28] Feng, H.; Hu, H.; Dong, H.; Xiao, Y.; Cai, Y.; Lei, B.; Liu, Y.; Zheng, M. J. Power Sources 2016, 302, 164.
[29] Liu, C.; Wang, J.; Li, J.; Zeng, M.; Luo, R.; Shen, J.; Sun, X.; Han, W.; Wang, L. ACS Appl. Mater. Interfaces 2016, 8, 7194.
[30] Xing, W.; Qiao, S. Z.; Ding, R. G.; Li, F.; Lu, G. Q.; Yan, Z. F.; Cheng, H. M. Carbon 2016, 44, 216.
[31] Ling, Z.; Wang, Z.; Zhang, M.; Yu, C.; Wang, G.; Dong, Y.; Liu, S.; Wang, Y.; Qiu, J. Adv. Funct. Mater. 2016, 26, 111.
/
| 〈 |
|
〉 |