

一维隐钾锰矿型二氧化锰的控制合成及其电化学性能
Controllable Synthesis of One-dimensional Cryptomelane-type Manganese Dioxide and Its Electrochemical Performance
Received date: 2017-09-13
Online published: 2018-01-09
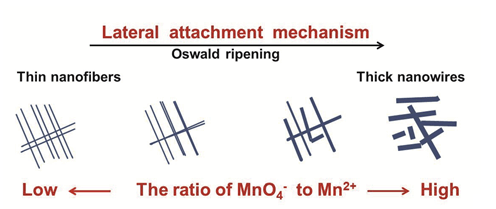
在不加酸条件下,通过调节高锰酸钾和一水硫酸锰的比率,合成了一系列正八面体分子筛二氧化锰纳米材料,并且实现了对一维OMS-2纳米材料直径的有效调控.氮气吸脱附分析(BET),傅里叶变换红外光谱(FT-IR),X射线衍射(XRD),透射电镜(TEM)和氢气程序升温还原(H2-TPR)的详细表征表明:一维二氧化锰纳米材料主要遵循侧向生长机制,奥斯瓦尔德熟化的作用是使两条或多条初始纳米纤维融合在一起.MnO6正八面体中阳离子Mn3+能促进一维二氧化锰纳米材料的纵向生长,而Mn4+则倾向于促进侧向生长.电化学测试表明,一维OMS-2纳米材料的直径强烈影响其比电容,最小直径材料的比电容为375 F/g.
张成明 , 庞鑫 , 王永钊 . 一维隐钾锰矿型二氧化锰的控制合成及其电化学性能[J]. 化学学报, 2018 , 76(2) : 133 -137 . DOI: 10.6023/A17090418
Cryptomelane-type manganese dioxide (OMS-2) is a very important nanomaterial in electrochemistry. Its intrinsic properties can be tailored by controlling shape or size. The diameter of one-dimensional OMS-2 nanomaterial is an important parameter in controllable synthesis and electrochemistry applications. Generally, the control of the diameter of one-dimensional OMS-2 nanomaterial can be realized by cosolvents or surfactants, even other special methods. In this paper, without any acid added, a series of one-dimensional OMS-2 nanomaterial with different diameters were synthesized by adjusting the ratio of potassium permanganate to manganese sulfate monohydrate in the aqueous solution with the conditional reflux method. The typical samples were characterized in detail by N2 adsorption-desorption analyses (BET), Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), transmission electron microscope (TEM) and hydrogen temperature-programmed reduction (H2-TPR). The results reconfirmed that the growth of OMS-2 nanofibers and nanowires mainly followed the lateral attachment mechanism. The role of Oswald ripening in the growth of one-dimensional OMS-2 nanomaterials was making two or more primary thinner nanofibers or nanowires welded together. In the synthesis process, all the conditions were strictly controlled. The samples synthesized at low ratio of MnO4- to Mn2+ showed thinner and longer nanofibers or nanowires, and the samples synthesized at high ratio of MnO4- to Mn2+ exhibited higher diameter. Therefore, it can be concluded that MnO4- can promote the lateral growth of one-dimensional OMS-2 nanomaterials and Mn2+ tends to promote the longitudinal growth. In the electrochemical tests, when the ratio of potassium permanganate to manganese sulfate monohydrate increased from 0.15 to 1.80, the specific capacitance of one-dimensional OSM-2 nanomaterials decreased gradually. Therefore, the specific capacitance of one-dimensional OSM-2 nanomaterial was directly related to their diameters. The smaller the diameter is, the larger the capacitance is. The specific capacitance of MnO-15, MnO-45, MnO-112 and MnO-180 was 375, 230, 144 and 77 F/g, respectively. The result of galvanostatic charge and discharge of four samples at the current density of 1 A/g in 1 mol/L Na2SO4 solution was consistent with the cyclic voltammetry.

Key words: manganese oxide; one-dimensional; diameter; electrochemistry
[1] Thenuwara, A. C.; Shumlas, S. L.; Attanayake, N. H.; Cerkez, E. B.; McKendry, I. G.; Frazer, L.; Borguet, E.; Kang, Q.; Zdilla, M. J.; Sun, J. Langmuir 2015, 31, 12807.
[2] Liu, J.; Younesi, R.; Gustafsson, T.; Edström, K.; Zhu, J. Nano Energy 2014, 10, 19.
[3] Ran, F.; Fan, H.; Wang, L.; Zhao, L.; Tan, Y.; Zhang, X.; Kong, L.; Kang, L. J. Energ. Chem. 2013, 22, 928.
[4] Zhang, K.; Han, P.; Gu, L.; Zhang, L.; Liu, Z.; Kong, Q.; Zhang, C.; Dong, S.; Zhang, Z.; Yao, J.; Xu, H.; Cui, G.; Chen, L. ACS Appl. Mater. Interfaces 2012, 4, 658.
[5] Hu, B.; Chen, C. H.; Frueh, S. J.; Jin, L.; Joesten, R.; Suib, S. L. J. Phys. Chem. C 2010, 114, 9835.
[6] Yang, C.; Gong, Z.; Zhao, W.; Yang, Y. Acta Chim. Sinica 2017, 75, 212(in Chinese). (杨春, 龚正良, 赵文高, 杨勇, 化学学报, 2017, 75, 212.)
[7] Liu, L.; Qi, X.; Hu, Y.; Chen, L.; Huang, X. Acta Chim. Sinica 2017, 75, 218(in Chinese). (刘丽露, 戚兴国, 胡勇胜, 陈立泉, 黄学杰, 化学学报, 2017, 75, 218.)
[8] Brock, S. L.; Duan, T. Z. R. Chem. Mater. 1998, 10, 2619.
[9] Su, Z.;Ye, S.; Wang, Y. Acta Chim. Sinica 2009, 67, 2413(in Chinese). (粟智, 叶世海, 王永龙, 化学学报, 2009, 67, 2413.)
[10] Debart, A.; Paterson, A. J.; Bao, J.; Bruce, P. G. Angew. Chem. Int. Ed. 2008, 47, 4521.
[11] Nyutu, E. K.; Chen, C. H.; Sithambaram, S.; Crisostomo,V. M. B.; Suib, S. L. J. Phys. Chem. C 2008, 112, 6786.
[12] Kumar, N.; Dineshkumar, P.; Rameshbabu, R.; Sen, A. Mater. Lett. 2015, 158, 309.
[13] Sampanthar, J. T.; Dou, J.; Joo, G. G.; Widjaja, E.; Eunice, L. Q. H. Nanotechnology 2007, 18, 25601.
[14] Xu, N.; Ma, X.; Qiao, S.; Yuan, J.; Liu, Z. Acta Chim. Sinica 2009, 67, 2566(in Chinese). (许乃才, 马向荣, 乔山峰, 袁佳琦, 刘宗怀, 化学学报, 2009, 67, 2566.)
[15] Hernández, W. Y.; Centeno, M. A.; Romero Sarria, F.; Ivanova, S.; Montes, M.; Odriozola, J. A. Catal. Today 2010, 157, 160.
[16] Li, Y.; Wang, J.; Zhang, Y.; Banis, M. N.; Liu, J.; Geng, D.; Li, R.; Sun, X. J. Colloid. Interf. Sci. 2012, 369, 123.
[17] Sun, H.; Liu, Z.; Chen, S.; Quan, X. Chem. Eng. J. 2015, 270, 58.
[18] Gao, T.; Glerup, M.; Krumeich, F.; Nesper, R.; Fjellvåg, H.; Norby, P. J. Phys. Chem. C 2008, 112, 13134.
[19] Ananth, M. V.; Pethkar, S.; Dakshinamurthi, K. J. Power Sources 1998, 75, 278.
[20] Portehault, D.; Cassaignon, S.; Baudrin, E.; Jolivet, J. P. Chem. Mater. 2007, 19, 5410.
[21] Hou, J.; Liu, L.; Li, Y.; Mao, M.; Lv, H.; Zhao, X. Environ. Sci. Technol. 2013, 47, 13730.
[22] Tang, W.; Shan, X.; Li, S.; Liu, H.; Wu, X.; Chen, Y. Mater. Lett. 2014, 132, 317.
[23] Carno, J.; Ferrandon, M.; Bjrnbom, E.; Jaras, S. Appl. Catal. A:Gen. 1997, 155, 265.
[24] Ivanova, S.; Petit, C.; Pitchon, V. Appl. Catal. A:Gen. 2007, 267, 191.
[25] Gac, W. Appl. Catal. B:Environ. 2007, 75, 107.
[26] Zhang, X.; Sun, X.; Zhang, H.; Li, C.; Ma, Y. Electrochim. Acta 2014, 132, 315.
[27] Chai, C.; Liu, A.; Lv, Y.; Mu, J.; Zhang, X.; Che, H. Mater. Lett. 2017, 196, 308.
[28] Wan, C.; Wang, L.; Shen, S.; Zhu, X. Acta Chim. Sinica 2009, 67, 1559(in Chinese). (万传云, 王利军, 沈绍典, 朱贤, 化学学报, 2009, 67, 1559.)
[29] Dong, S.; Chen, X.; Gu, L.; Zhou, X.; Li, L.; Liu, Z.; Han, P.; Xu, H.; Yao, J.; Wang, H.; Zhang, X.; Shang, C.; Cui, G.; Chen, L. Energy Environ. Sci. 2011, 4, 3502.
/
| 〈 |
|
〉 |