

通过远端碳氮双键迁移实现非活化烯烃的三氟甲硫基化反应
收稿日期: 2018-08-02
网络出版日期: 2018-08-27
基金资助
项目受国家自然科学基金(No.21722205)资助.
Difunctionalization of Unactivated Alkenes through SCF3 Radical-triggered Distal Functional Group Migration
Received date: 2018-08-02
Online published: 2018-08-27
Supported by
Project supported by the National Natural Science Foundation of China (No. 21722205).
陈栋 , 吉梅山 , 姚英明 , 朱晨 . 通过远端碳氮双键迁移实现非活化烯烃的三氟甲硫基化反应[J]. 化学学报, 2018 , 76(12) : 951 -955 . DOI: 10.6023/A18080313
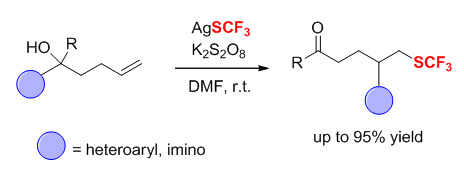
Radical-mediated C-SCF3 bond formation via the addition of SCF3 radical to alkenes has become an efficient strategy for the construction of alkyl trifluoromethylthioethers. However, the scope of alkenes is largely limited to activated alkenes in which the presence of adjacent carbonyl or aryl group is required to stabilize the alkyl radical intermediates by p-π conjugation. A few cases involving trifluoromethylthiolation of unactivated olefins have been reported, but in these reactions only a single functional group is incorporated to alkenes. The radical difunctionalization of unactivated olefins remains challenging and has received less attention. Recently, we established a new protocol to realize the radical difunctionalization of alkenes through intramolecularly distal functional group migration. This tactic provides a useful and elegant tool for the elusive functionalization of unactivated olefins. A portfolio of groups such as cyano, heteroaryl, imino, aldehyde, and alkynyl can be readily migrated in the transformation. Herein, we disclose an efficient and practical approach for the trifluoromethylthiolation of unactivated olefins based on the intramolecular migration of heteroaryl and imino groups. The migration is triggered by the addition of SCF3 radical, which is generated from the mixture of AgSCF3 and K2S2O8at room temperature, to alkenes. The reaction demonstrates a high functional group compatibility and broad substrate scope. A variety of nitrogen-containing five- and six-membered heteroaryl as well as imino groups are readily migrated, affording the synthetically valuable alkyl trifluoromethylthioether compounds in good yields. The typical procedure is as follows: a mixture of tertiary alcohol (0.2 mmol), AgSCF3(0.3 mmol), and K2S2O8(0.6 mmol) is loaded in a flame-dried reaction vial which is subjected to evacuation/flushing with nitrogen three times. Dry DMF (2.0 mL) is added to the mixture via syringe, and the mixture is then stirred at room temperature until the starting material is consumed which is determined by TLC. The mixture is extracted with ethyl acetate (10 mL×3). The combined organic extracts are washed with brine, dried over Na2SO4, filtered, concentrated, and purified by flash column chromatography on silica gel (eluent: petroleum ether/ethyl acetate) to give the desired product.
[1] (a) Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525;
(b) Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165.
[2] (a) Leroux, F.; Jeschke, P.; Schlosser, M. Chem. Rev. 2005, 105, 827;
(b) Manteau, B.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. J. Fluorine Chem. 2010, 131, 140.
[3] (a) Boiko, V. N. Beilstein J. Org. Chem. 2010, 6, 880;
(b) Landelle, G.; Panossian, A.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. Beilstein J. Org. Chem. 2013, 9, 2476;
(c) Liang, T.; Neumann, C. N.; Ritter, T. Angew. Chem., Int. Ed. 2013, 52, 8214;
(d) Tlili, A.; Billard, T. Angew. Chem., Int. Ed. 2013, 52, 6818;
(e) Toulgoat, F.; Alazet, S.; Billard, T. Eur. J. Org. Chem. 2014, 2415;
(f) Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731;
(g) He, W.; Weng, Z. Prog. Chem. 2013, 25, 1071(in Chinese). (何伟明, 翁志强, 化学进展, 2013, 25, 1071);
(h) Xu, J.; Chen, P.; Ye, J.; Liu, G. Acta Chim. Sinica 2015, 73, 1294(in Chinese). (徐佳斌, 陈品红, 叶金星, 刘国生, 化学学报, 2015, 73, 1294);
(i) Zhang, K.; Xu, X.; Qing, F. Chin. J. Org. Chem. 2015, 35, 556(in Chinese). (张柯, 徐修华, 卿凤翎, 有机化学, 2015, 35, 556);
(j) Zhang, P.; Lv, L.; Shen, Q. Acta Chim. Sinica 2017, 75, 744(in Chinese). (张盼盼, 吕龙, 沈其龙, 化学学报, 2017, 75, 744);
(k) Hui, R.; Zhang, S.; Tan, Z.; Wu, X.; Feng, B. Chin. J. Org. Chem. 2017, 37, 3060(in Chinese). (惠人杰, 张士伟, 谭政, 吴小培, 冯柏年, 有机化学, 2017, 37, 3060);
(l) Zhao, X.; Li, T.; Tian, M.; Su, Z.; Wei, A.; Lu, K. Chin. J. Org. Chem. 2018, 38, 677(in Chinese). (赵霞, 李天娇, 田苗苗, 苏志扬, 魏奥琪, 芦逵, 有机化学, 2018, 38, 677).
[4] (a) Ferry, A.; Billard, T.; Langlois, B. R.; Bacqué, E. Angew. Chem., Int. Ed. 2009, 48, 8551;
(b) Zhang, P.; Li, M.; Xue, X.-S.; Xu, C.; Zhao, Q.; Liu, Y.; Wang, H.; Guo, Y.; Lu, L.; Shen, Q. J. Org. Chem. 2016, 81, 7486.
[5] Yin, F.; Wang, X.-S. Org. Lett. 2014, 16, 1128.
[6] Fuentes, N.; Kong, W.; Fernández-Sánchez, L.; Merino, E.; Nevado, C. J. Am. Chem. Soc. 2015, 137, 964.
[7] Honeker, R.; Garza-Sanchez, R. A.; Hopkinson, M. N.; Glorius, F. Chem. Eur. J. 2016, 22, 4395.
[8] Zhang, K.; Liu, J.-B.; Qing, F.-L. Chem. Commun. 2014, 50, 14157.
[9] Yang, T.; Lu, L.; Shen, Q. Chem. Commun. 2015, 51, 5479.
[10] (a) Pintauer, T.; Matyjaszewski, K. Chem. Soc. Rev. 2008, 37, 1087;
(b) Eckenhoff, W. T.; Pintauer, T. Catal. Rev. 2010, 52, 1;
(c) Cao, M.-Y.; Ren, X.; Lu, Z. Tetrahedron Lett. 2015, 56, 3732;
(d) Clark, A. J. Eur. J. Org. Chem. 2016, 2231;
(e) Kindt, S.; Heinrich, M. R. Synthesis 2016, 48, 1597.
[11] (a) Wu, Z.; Ren, R.; Zhu, C. Angew. Chem., Int. Ed. 2016, 55, 10821;
(b) Ji, M.; Wu, Z.; Yu, J.; Wan, X.; Zhu, C. Adv. Synth. Catal. 2017, 359, 1959;
(c) Ren, R.; Wu, Z.; Huan, L.; Zhu, C. Adv. Synth. Catal. 2017, 359, 3052;
(d) Ji, M.; Yu, J.; Zhu, C. Chem. Commun. 2018, 54, 6812.
[12] (a) Wu, Z.; Wang, D.; Liu, Y.; Huan, L.; Zhu, C. J. Am. Chem. Soc. 2017, 139, 1388;
(b) Wu, X.; Wang, M.; Huan, L.; Wang, D.; Wang, J.; Zhu, C. Angew. Chem., Int. Ed. 2018, 57, 1640;
(c) Wang, M.; Wu, Z.; Zhang, B.; Zhu, C. Org. Chem. Front. 2018, 5, 1896;
(d) Chen, D.; Wu, Z.; Yao, Y.; Zhu, C. Org. Chem. Front. 2018, 5, 2370;
(e) Zhang, H.; Wu, X.; Zhao, Q.; Zhu, C. Chem. Asian J. 2018, DOI:10.1002/asia.201800150.
[13] Yu, J.; Wang, D.; Xu, Y.; Wu, Z.; Zhu, C. Adv. Synth. Catal. 2018, 360, 744.
[14] Xu, Y.; Wu, Z.; Jiang, J.; Ke, Z.; Zhu, C. Angew. Chem., Int. Ed. 2017, 56, 4545.
[15] For selected reviews, see:(a) Wu, X.; Wu, S.; Zhu, C. Tetrahedron Lett. 2018, 59, 1328;
(b) Li, W.; Xu, W.; Xie, J.; Yu, S.; Zhu, C. Chem. Soc. Rev. 2018, 47, 654.
[16] (a) Thaharn, W.; Soorukram, D.; Kuhakarn, C.; Tuchinda, P.; Reutrakul, V.; Pohmakotr, M. Angew. Chem., Int. Ed. 2014, 53, 2212;
(b) Kong, W.; Casimiro, M.; Merino, E.; Nevado, C. J. Am. Chem. Soc. 2013, 135, 14480;
(c) Kong, W.; Merino; E;. Nevado, C. Angew. Chem., Int. Ed. 2014, 53, 5078;
(d) Fuentes, N.; Kong, W.; Fernandez-Sanchez, L.; Merino, E.; Nevado, C. J. Am. Chem. Soc. 2015, 137, 964;
(e) Kong, W.; Fuentes, N.; Garcia-Dominguez, A.; Merino, E.; Nevado, C. Angew. Chem., Int. Ed. 2015, 54, 2487;
(f) Zhou, T.; Luo, F.-X.; Yang, M.; Shi, Z.-J. J. Am. Chem. Soc. 2015, 137, 14586;
(g) Li, Z.-L.; Li, X.-H.; Wang, N.; Yang, N.-Y.; Liu, X.-Y. Angew. Chem., Int. Ed. 2016, 55, 15100;
(h) Li, L.; Li, Z.-L.; Wang, F.-L.; Guo, Z.; Cheng, Y.-F.; Wang, N.; Dong, X.-W.; Fang, C.; Liu, J.; Hou, C.; Tan, B.; Liu, X.-Y. Nat. Commun. 2016, 7, 13852;
(i) Li, L.; Gu, Q.-S.; Wang, N.; Song, P.; Li, Z.-L.; Li, X.-H.; Wang, F.-L.; Liu, X.-Y. Chem. Commun. 2017, 53, 4038;
(j) Wang, N.; Li, L.; Li, Z.-L.; Yang, N.-Y.; Guo, Z.; Zhang, H.-X.; Liu, X.-Y. Org. Lett. 2016, 18, 6026;
(k) Gu, L.; Gao, Y.; Ai, X.; He, C. Y.; Li, G.; Yuan, M. Chem. Commun. 2017, 53, 12946;
(l) Tang, X.; Studer, A. Chem. Sci. 2017, 8, 6888;
(m) Tang, X.; Studer, A. Angew. Chem., Int. Ed. 2018, 57, 814;
(n) Li, L.; Li, Z.-L.; Gu, Q.-S.; Wang, N.; Liu, X.-Y. Sci. Adv. 2017, 3, e1701487;
(o) Liu, J.; Li, W.; Xie, J.; Zhu, C. Org. Chem. Front. 2018, 5, 797;
(p) Zhao, Q.; Ji, X.-S.; Gao, Y.-Y.; Hao, W.-J.; Zhang, K.-Y.; Tu, S.-J.; Jiang, B. Org. Lett. 2018, 20, 3596;
(q) Wang, H.; Xu, Q.; Yu, S. Org. Chem. Front. 2018, 5, 2224;
(r) Wei, X.-J.; Noël, T. J. Org. Chem. 2018, DOI:10.1021/acs.joc.8b01624.
/
| 〈 |
|
〉 |