

碳-氟键断裂用于构建氟代异噁唑化合物
收稿日期: 2018-07-17
网络出版日期: 2018-09-14
基金资助
项目受国家自然科学基金(Nos.21472137,21532008,21772142)和国家重点基础研究发展计划(973计划,No.2014CB745100)资助.
Facile Synthesis of Fluorinated Isoxazoles via Consecutive Double C—F Bond Cleavage
Received date: 2018-07-17
Online published: 2018-09-14
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21472137, 21532008, 21772142) and the National Basic Research Program of China (973 Program, No. 2014CB745100).
任智雯 , 任楠 , 张发光 , 马军安 . 碳-氟键断裂用于构建氟代异噁唑化合物[J]. 化学学报, 2018 , 76(12) : 940 -944 . DOI: 10.6023/A18070279
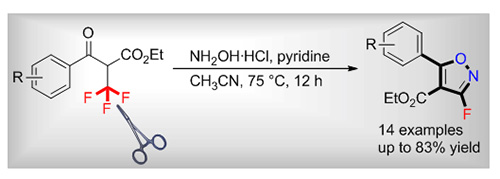
Fluorinated heterocycles represent a ubiquitous structural motif found in numerous pharmaceuticals, agrochemicals, and functional materials. This is especially true for fluorine-containing five-membered heteroaromatic compounds that have been widely investigated in various fields for a long time. In this context, fluorinated isoxazoles have emerged as valuable scaffolds owing to their diverse biological properties. Among various approaches that have been developed for the synthesis and functionalization of isoxazoles, efficient and modular route to fluorine-substituted isoxazoles are still limited. Traditional methods include the condensation of 2-fluoro-1,3-dicarbonyl derivatives with hydroxylamine, Au-catalyzed fluorocyclization of 2-alkyne O-methyloximes, and direct fluorination of isoxazoles. However, the wide applicability of these approaches often suffers from low chemical yields, harsh reaction conditions, and limited substrate scope. Herein, we describe a one-pot protocol for the construction of fluorinated isoxazoles from CF3-containing precursors with hydroxylammonium chloride. Typical features of this reaction include mild conditions, simple operations, and good functional group compatibility. This method provides facile access to a series of 3-F-5-aryl-isoxazoles in moderate to good yields from easily available α-CF3-β-keto esters. Moreover, further synthetic transformations of obtained isoxazoles to important bio-active molecular derivatives have also been demonstrated. A representative procedure for this reaction is as following: α-CF3-β-keto ester 1 (0.2 mmol, 1.0 equiv.), HONH2·HCl (46 mg, 0.66 mmol), pyridine (71 μL, 0.88 mmol), and CH3CN (3.0 mL) were added into an oven-dried vial equipped with a magnetic stir bar. The mixture was stirred at 75 ℃ for 12 h and monitored by thin-layer chromatography (TLC). After completion, 10 mL of water was added and the mixture was extracted with EtOAc for three times. The combined organic layers were washed with saturated NaCl and dried over Na2SO4. The mixture was evaporated under reduced pressure and residue was purified by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (V:V=30:1) to afford the 3-F-5-aryl-isoxazole 2.
[1] (a) Schlosser, M. Angew. Chem., Int. Ed. 2006, 45, 5432.
(b) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
(c) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359.
(d) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
(e) Ma, J.-A.; Cahard, D. Chem. Rev. 2008, 108, PR1.
(f) Fluorinated Hetreocycles, ACS Symp. Ser. No. 1003, Eds.:Gakh, A. A.; Kirk, K. L. ACS, Washington, DC, 2009, pp. 3~20.
(g) Furuya, T.; Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470.
(h) Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Chem. Soc. Rev. 2011, 40, 3496.
(i) Li, S.; Ma, J.-A. Chem. Soc. Rev. 2015, 44, 7439.
(j) Jeschke, P. Pest Manage. Sci. 2017, 73, 10536.
(k) Chen, C.; Fu, L.; Chen, P.; Liu, G. Chin. J. Chem. 2017, 35, 1781.
(l) Yang, Q.-L.; Fang, P.; Mei, T.-S. Chin. J. Chem. 2018, 36, 338.
[2] (a) Fluorine in Heterocyclic Chemistry, Vol. 1, Eds.:Nenajdenko, V., Springer International Publishing, 2014.
(b) Giornal, F.; Pazenok, S.; Rodefeld, L.; Lui, N.; Vors, J.-P.; Leroux, F. R. J. Fluorine Chem. 2013, 152, 2.
[3] (a) Cordero, F. M.; Giomi, D.; Lascialfari, L. In Progress in Heterocyclic Chemistry, Vol. 27, Eds.:Gribble, G. W.; Joule, J. A. Elsevier Ltd., 2015, p. 321.
(b) Agrawal, N.; Mishra, P. Med. Chem. Res. 2018, 27, 1309.
[4] (a) Pinho, M.; Teresa, M. V. D. Curr. Org. Chem. 2005, 9, 925.
(b) Kaur, K.; Kumar, V.; Sharma, A. K.; Gupta, G. K. Eur. J. Med. Chem. 2014, 77, 121.
(c) Hu, F.; Szostak, M. Adv. Synth. Catal. 2015, 357, 2583.
(d) Morita, T.; Yugandar, S.; Fuse, S.; Nakamura, H. Tetrahedron Lett. 2018, 59, 1159.
[5] (a) Fluorine in Medicinal Chemistry and Chemical Biology, Eds.:Ojima, I., Wiley-Blackwell, Chichester, UK, 2009.
(b) Isanbora, C.; O'Hagan, D. J. Fluorine Chem. 2006, 127, 303.
[6] (a) Peng, W.-M.; Zhu, S.-Z. Acta Chim. Sinica 2003, 61, 455. (彭卫民, 朱仕正, 化学学报, 2003, 61, 455.)
(b) Kumar, V.; Kaur, K. J. Fluorine Chem. 2015, 180, 55.
[7] (a) Bumgardner, C. L.; Sloop, J. C. J. Fluorine Chem. 1992, 56, 141.
(b) Sloop, J. C.; Bumgardner, C. L.; Loehle, W. D. J. Fluorine Chem. 2002, 118, 135.
[8] (a) Stephens, C. E.; Blake, J. A. J. Fluorine Chem. 2004, 125, 1939.
(b) Sato, K.; Sandford, G.; Shimizu, K.; Akiyama, S.; Lancashire, M. J.; Yufit, D. S.; Tarui, A.; Omote, M.; Kumadaki, I.; Harusawa, S.; Ando, A. Tetrahedron 2016, 72, 1690.
[9] Jeong, Y.; Kim, B.-I.; Lee, J. K.; Ryu, J.-S. J. Org. Chem. 2014, 79, 6444.
[10] (a) Dighe, S. U.; Mukhopadhyay, S.; Kolle, S.; Kanojiya, S.; Batra, S. Angew. Chem., Int. Ed. 2015, 54, 10926.
(b) Yuan, X.; Yao, J.-F.; Tang, Z.-Y. Org. Lett. 2017, 19, 1410.
[11] (a) Amii, H.; Uneyama, K. Chem. Rev. 2009, 109, 2119.
(b) Ahrens, T.; Kohlmann, J.; Ahrens, M.; Braun, T. Chem. Rev. 2015, 115, 931.
(c) Shen, Q.; Huang, Y.-G.; Liu, C.; Xiao, J.-C.; Chen, Q.-Y.; Guo, Y. J. Fluorine Chem. 2015, 179, 14.
[12] Ohtsuka, Y.; Uraguchi, D.; Yamamoto, K.; Tokuhisa, K.; Yamakawa, T. J. Fluorine Chem. 2016, 181, 1.
[13] Okamoto, K.; Nanya, A.; Eguchi, A.; Ohe, K. Angew. Chem., Int. Ed. 2018, 57, 1039.
[14] CCDC 1843944 contains the supplementary crystallographic data for this compound 2f, these data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.
[15] (a) Watterson, S. H.; Guo, J.; Spergel, S. H.; Langevine, C. M.; Moquin, R. V.; Shen, D. R.; Yarde, M.; Cvijic, M. E.; Banas, D.; Liu, R.; Suchard, S. J.; Gillooly, K.; Taylor, T.; Rex-Rabe, S.; Shuster, D. J.; McIntyre, K. W.; Cornelius, G.; D'Arienzo, C.; Marino, A.; Balimane, P.; Warrack, B.; Salter-Cid, L.; McKinnon, M.; Barrish, J. C.; Carter, P. H.; Pitts, W. J.; Xie, J.; Dyckman, A. J. J. Med. Chem. 2016, 59, 2820.
(b) Hou, X.; Zhang, H.; Chen, B.-C.; Guo, Z.; Singh, A.; Goswami, A.; Gilmore, J. L.; Sheppeck, J. E.; Dyckman, A. J.; Carter, P. H.; Mathur, A. Org. Process Res. Dev. 2017, 21, 200.
[16] (a) Menozzi, G.; Schenone, P.; Mosti, L. J. Heterocycl. Chem. 1983, 20, 645.
(b) Strah, S.; Golobic, A.; Golobic, L.; Stanovnik, B. J. Heterocycl. Chem. 1997, 34, 1511.
(c) Tang, X.-H.; Hu, C.-M. J. Fluorine Chem. 1995, 74, 9.
(d) Ohtsuka, Y.; Uraguchi, D.; Yamamoto, K.; Tokuhisa, K.; Yamakawa, T. Tetrahedron 2012, 68, 2636.
(e) Xu, L.; Zhang, Q.; Xie, Q.; Huang, B.; Dai, J.-J.; Xu, J.; Xu, H.-J. Chem. Commun. 2018, 54, 4406.
/
| 〈 |
|
〉 |