

高活性单分散磺化聚苯乙烯多孔微球用于生物柴油制备研究
收稿日期: 2018-08-15
网络出版日期: 2018-10-08
基金资助
项目受国家自然科学基金(No.21802039)、国家级大学生创新创业训练计划(教高司函[2017]40号)、湖南省大学生研究性学习和创新性实验计划(湘教通[2017]205号,湘教通[2018]255号)和湖南省重点建设学科(湘教发[2011]76号)资助.
Study on High Activity Monodispersed Sulfonated Porous Polystyrene Microspheres for Preparation of Biodiesel
Received date: 2018-08-15
Online published: 2018-10-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 21802039), the National College Students' Innovation and Entrepreneurship Training Program Project (Jiao Gao Si No.[2017]40), Hunan Province College Students' Research Learning and Innovative Experiment Project (Xiang Jiao Tong Nos.[2017]205,[2018]255) and the Construct Program of the Key Discipline in Hunan Province (Xiang Jiao Fa No.[2011]76).
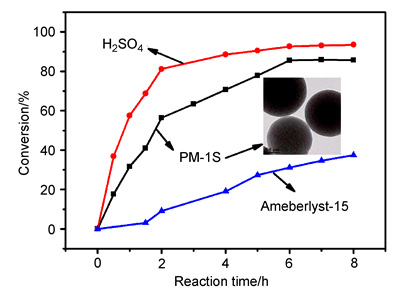
采用回流-沉淀聚合法高效制备了单分散交联多孔聚苯乙烯微球,再在温和的条件下对其进行磺化,制备了磺化聚苯乙烯多孔微球.以调控磺化度和酸密度为目标,重点考察了溶剂用量、溶胀时间、氯磺酸用量、磺化温度和磺化时间等影响因素.用5 mL CCl4溶胀0.5 g交联聚苯乙烯微球,然后加入0.3 mL氯磺酸,在50℃磺化75 min,磺化度和酸密度分别可以达到85.1%和2.611 mmol·g-1.该磺化聚苯乙烯微球在催化油酸和甲醇酯化合成生物柴油中表现出很高的催化活性,远高于酸性离子交换树脂Ameberlyst-15,接近于均相的浓硫酸体系;且循环使用3次后,催化活性仍可保持初始活性的92%.提供了一种简单、绿色和可控的方法,制备了酸密度可控、稳定性好的单分散磺化聚苯乙烯微球,在工业制备生物柴油领域具有较好的前景.
罗建新 , 颜文海 , 马青 , 张春燕 , 方怡权 , 张栩诚 , 汪长春 . 高活性单分散磺化聚苯乙烯多孔微球用于生物柴油制备研究[J]. 化学学报, 2019 , 77(1) : 54 -59 . DOI: 10.6023/A18080335
Sulfonated porous polystyrene microspheres as heterogeneous catalyst for preparation of biodiesel were prepared by a facile two-step synthesis process in this work. Firstly, monodisperse porous cross-linked polystyrene microspheres were prepared by reflux-precipitation polymerization, in which different ratio of styrene (St) and divinylbenzene (DVB) were used as monomer and cross-linker, respectively. Then the obtained polystyrene microspheres were sulfonated by chlorosulfonic acid. The structure and composition of the related polystyrene microspheres were characterized by FT-IR, X-ray photoelectron spectroscopy, elemental analysis, thermogravimetric analysis, Brunauer-Emmett-Teller (BET) surface area analysis, transmission electron microscope, particle size and zeta potential analysis. In order to adjust the sulfonated degree and acid density, the reaction parameters such as solvent dosage, swelling time, amount of chlorinated sulfonic acid, sulfonated temperature and sulfonated time were investigated carefully. The optimum conditions for the sulfonated reaction are as follows:0.5 g of cross-linked polystyrene microspheres was swollen with 5 mL of CCl4, and then sulfonated with 0.3 mL of chlorosulfonic acid at 50℃ for 75 min. In addition, the effect of crosslinking degree on the specific surface area and acid density of the sulfonated polystyrene microspheres were also studied. The as-prepared sulfonated polystyrene microspheres exhibited high acid density (2.611 mmol·g-1), good thermal stability (up to about 200℃) and appropriate specific surface area. The sulfonated porous polystyrene microspheres as catalyst were applied in the esterification reaction of oleic acid and methanol. Our experimental results showed that the functional particles possessed very high catalytic activity, which was much higher than polystyrene ion exchange resin (Ameberlyst-15) and close to concentrated sulfuric acid. The catalytic activity still maintained 92% of the initial activity after 3 runs of recycling use, and the shedding of sulfonic acid groups was almost negligible. This paper demonstrates a simple, green and controllable strategy to develop sulfonated porous polystyrene microspheres with high catalytic activity and good durability for preparation of biodiesel.

[1] Pirola, C.; Bianchi, C. L.; Boffito, D. C.; Carvoli, G.; Ragaini, V. Ind. Eng. Chem. Res. 2010, 49, 4601.
[2] Cheng, J.; Qiu, Y.; Huang, R.; Yang, W.; Zhou, J.; Cen, K. Bioresour. Technol. 2016, 221, 344.
[3] Shu, Q.; Hou, X. P.; Tang, G. Q.; Liu, F. S.; Yuan, H.; Xu, B. Q.; Zhang, C. X.; Wang, J. F. Chin. J. Inorg. Chem. 2016, 32, 1791. (舒庆, 侯小鹏, 唐国强, 刘峰生, 袁红, 许宝泉, 张彩霞, 王金福, 无机化学学报, 2016, 32, 1791.)
[4] Liu, F. J.; Huang, K.; Zheng, A. M.; Xiao, F. S.; Dai, S. ACS Catal. 2018, 8, 372.
[5] Lee, A. F.; Bennett, J. A.; Manayil, J. C.; Wilson, K. Chem. Soc. Rev. 2014, 43, 7887.
[6] De, S.; Dutta, S.; Saha, B. Catal. Sci. Technol. 2016, 6, 7364.
[7] Gupta, P.; Paul, S. Catal. Today 2014, 236, 153.
[8] Lian, Y. F.; Yan, L. L.; Wang, Y.; Qi, X. H. Acta Chim. Sinica 2014, 72, 502. (廉优芬, 闫碌碌, 王羽, 漆新华, 化学学报, 2014, 72, 502.)
[9] Akiyama, G.; Matsuda, R.; Sato, H.; Takata, M.; Kitagawa, S. Adv. Mater. 2011, 23, 3294.
[10] Wang, Z. Q.; Liu, H. Y.; Cui, H. L.; Zhang, M. H.; Zhang, Z. B. Ind. Eng. Chem. Res. 2015, 54, 7219.
[11] Yu, F.; Smet, M.; Dehaen, W.; Sels, B. F. Chem. Commun. 2016, 52, 2756.
[12] Liu, F. J.; Kong, W. P.; Qi, C. Z.; Zhu, L. F.; Xiao, F. S. ACS Catal. 2012, 2, 565.
[13] Zhang, X. M.; Zhao, Y. P.; Xu, S. T.; Yang, Y.; Liu, J.; Wei, Y. X.; Yang, Q. H. Nat. Commun. 2014, 5, 3170.
[14] Jin, S.; Pan, Y.; Wang, C. C. Acta Chim. Sinica 2013, 71, 1500. (金莎, 潘元佳, 汪长春, 化学学报, 2013, 71, 1500.)
[15] Zhou, R.; Wei, R. Q.; Liu, X. N.; Lin, X. J. Chem. Ind. Eng. 2010, 61, 1047. (周蕊, 魏荣卿, 刘晓宁, 林弦, 化工学报, 2010, 61, 1047.)
[16] Ma, H.; Li, J. B.; Liu, W. W.; Cheng, B. J.; Cao, X. Y.; Mao, J. D.; Zhu, S. W. J. Agric. Food Chem. 2014, 62, 5345.
[17] Gao, Z. H.; Tang, S. K.; Cui, X. L.; Tian, S. J.; Zhang, M. H. Fuel 2015, 140, 669.
[18] Yu, H. W.; Niu, S. L.; Lu, C. M.; Li, J.; Yang, Y. Z. Fuel 2017, 208, 101.
[19] Yang, Q.; Pan, X. J. ACS Sustainable Chem. Eng. 2016, 4, 4824.
[20] Shu, Q.; Hou, X. P.; Zhu, L. H.; Shen, B. P.; Ma, F.; Wang, J. F. J. Fuel Chem. Technol. 2016, 44, 209. (舒庆, 侯小鹏, 朱丽华, 申邦坡, 马飞, 王金福, 燃料化学学报, 2016, 44, 209.)
[21] Li, M.; Chen, D. Y.; Zhu, X. F. Chin. J. Catal. 2013, 34, 1674.
/
| 〈 |
|
〉 |