

θ溶剂中树枝形均聚物的自洽场理论计算
收稿日期: 2018-08-27
网络出版日期: 2018-10-08
基金资助
项目受国家自然科学基金(Nos.21320102005,21774026)和中国人民共和国科学技术部(Nos.2016YFA0203301)资助.
Self-Consistent Field Theory of Dendritic Homopolymers in θ Solvent
Received date: 2018-08-27
Online published: 2018-10-08
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21320102005, 21774026) and Ministry of Science and Technology of the People's Republic of China (2016YFA0203301).
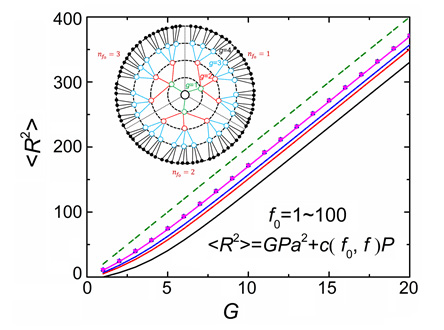
树枝形大分子因其高度支化的特殊结构,在传感、载药、催化等众多领域有着广泛的应用潜力.运用高斯链模型推导了溶液体系中树枝形均聚物的自洽场方程组.并在不考虑体积排除作用的条件下,计算了θ溶液中分子的中心链节官能度f0、支化点官能度f、间隔链段的聚合度P、以及总代数G对链节浓度分布及均方回转半径的影响.计算结果显示,链节浓度分布总是符合"dense-core"的模型,即在所有计算参数组合下,链节浓度总是沿径向单调下降,并随f0、f和G的增加而单调增加.自洽场计算得到的树枝形均聚物的均方回转半径R与Rouse动力学模型的计算结果基本一致,在f0、f和G的数值较大时,都能够得到<R2>≈GPa2的标度律关系.
付超 , 杨颖梓 , 邱枫 . θ溶剂中树枝形均聚物的自洽场理论计算[J]. 化学学报, 2019 , 77(1) : 95 -102 . DOI: 10.6023/A18080351
The dendrimers are a unique class of branched macromolecules with defined architectures synthesized by iterative reaction steps. Because of their highly branched structures, the dendrimers have a wide potential application in many fields, including sensing, drug delivery, catalysis, etc. In order to understand the thermal equilibrium behavior of the dendritic homopolymers in solution, we derived the self-consistent field theory (SCFT) for the dilute dendrimer solutions. The center segment is anchored on the origin of the space, and the shape of the dendrimer is assumed to be spherically symmetric. The pre-averaged interaction parameter u is employed to represent the volume exclusion interaction between the segments. We only focus on the dendrimer immersed in the θ solvent, where the volume exclusion interaction between the segments is negligible (u=0). The number density of the segments, φ(r), is calculated via systematically changing the topological parameters of the molecule, including the functionality f0 of the central segment, the functionality f of the branching points, the degree of polymerization of the spacers P, and the total generation number G. With all parameter combinations, φ(r) was found always maximized at the center and monotonically decreasing along the radial direction. Thus, the dendrimers in θ solvent obeys the "dense-core" model instead of the "dense-shell" model. Increasing f0, f and G results in the increase of φ(r) with any radius r. However, increasing P causes the decrease of φ(r) near the center region and the increase of φ(r) with larger r. The size of the dendrimer, analyzed by calculating the radius of gyration R, increases with f0, f, G and P. R calculated by our SCFT agrees well with the results obtained by the Rouse dynamics. With large f0, f and G, both SCFT and the Rouse dynamics predict the scaling law <R2>≈GPa2.

[1] Xia, K.; Sheng, R.; Zhu, Y.; Li, H.; Luo, T.; Xu, Y.; Cao, A. Acta Chim. Sinica 2010, 68, 1130(in Chinese). (夏可嘉, 盛瑞隆, 朱英丹, 李慧, 罗挺, 徐宇虹, 曹阿民, 化学学报, 2010, 68, 1130.)
[2] Li, J.; Zeng, Y.; Zhang, X.; Yu, T.; Chen, J.; Li, Y. Acta Chim. Sinica 2014, 72, 1157(in Chinese). (李婧, 曾毅, 张小辉, 于天君, 陈金平, 李嫕, 化学学报, 2014, 72, 1157.)
[3] Qin, T.; Zeng, Y.; Chen, J.; Yu, T.; Li, Y. Acta Chim. Sinica. 2017, 75, 99(in Chinese). (秦天依, 曾毅, 陈金平, 于天君, 李嫕, 化学学报, 2017, 55, 99.)
[4] Adachi, N.; Sugiyama, H.; Arai, M.; Ogawa, H. Molecules 2014, 19, 4135.
[5] Shaw, P. E.; Cavaye, H.; Chen, S. S. Y.; James, M.; Gentle, I. R.; Burn, P. L. Phys. Chem. Chem. Phys. 2013, 15, 9845.
[6] Rahman, M. A.; Noh, H. B.; Shim, Y. B. Anal. Chem. 2008, 80, 8020
[7] Peng, X. H.; Pan, Q. M.; Rempel, G. L. Chem. Soc. Rev. 2008, 37, 1619.
[8] Yoon, H. C.; Hong, M. Y.; Kim, H. S. Anal. Chem. 2000, 72, 4420.
[9] Bielinska, A. U.; Yen, A.; Wu, H. L.; Zahos, K. M.; Sun, R.; Weiner, N. D.; Baker, J. R.; Roessler, B. J. Biomaterials 2000, 21, 877.
[10] Vincent, L.; Varet, J.; Pille, J.-Y.; Bompais, H.; Opolon, P.; Maksimenko, A.; Malvy, C.; Mirshahi, M.; Lu, H.; Vannier, J.-P.; Soria, C.; Li, H. Int. J. Cancer 2003, 105, 419.
[11] Jain, S.; Kaur, A.; Puri, R.; Utreja, P.; Jain, A.; Bhide, M.; Ratnam, R.; Singh, V.; Patil, A. S.; Jayaraman, N.; Kaushik, G.; Yadav, S.; Khanduja, K. L. Eur. J. Med. Chem. 2010, 45, 4997.
[12] Kesharwani, P.; Jain, K.; Jain, K. N. Prog. Polym. Sci. 2014, 39, 268.
[13] Astruc, D.; Boisselier, E.; Ornelas, C. Chem. Rev. 2010, 110, 1857.
[14] Buhleier, G. E.; Wehner, W.; Vögtle, F. Chemischer Informationsdienst 1978, 2, 155.
[15] Cloninger, M. J. Curr. Opin. Chem. Biol. 2002, 6, 742.
[16] de Gennes, P. G.; Hervet, H. Journal de Physique-Lettres 1983, 44, L351.
[17] Lescanec, R. L.; Muthukumar, M. Macromolecules 1990, 23, 2280.
[18] Mansfield, M. L.; Klushin, L. I.; Klushint, L. I. Macromolecules 1993, 26, 4262.
[19] Biswas, P.; Cherayilk, B. J.; Klushint, L. I. J. Chem. Phys. 1993, 26, 4262.
[20] Chen, Z. Y.; Cui, S.-M. Macromolecules 1996, 29, 7943.
[21] Carl, M. J. Chem. Soc. 1996, 92, 4151.
[22] Carbone, P.; Müller-Plathe, F. Soft Matter 2009, 5, 2638.
[23] Jurjiu, A.; Dockhorn, N.; Mironova, O.; Sommer, J.-U. Soft Matter 2014, 10, 4935.
[24] Freire, J. J.; Rubio, A. M. Polymer 2008, 49, 2762.
[25] Boris, D.; Rubinstein, M. Macromolecules 1996, 29, 7251.
[26] Götze, I. O.; Likos, C. N. Macromolecules 2003, 36, 8189.
[27] Lyulin, A. V.; Davies, G. R.; Adolf, D. B. Macromolecules 2000, 33, 6899.
[28] Sheng, Y. J.; Jiang, S. Y.; Tsao, H. K. Macromolecules 2002, 35, 7865.
[29] Kumar, A.; Biswas, P. J. Chem. Phys. 2011, 134, 214901.
[30] Kumar, A.; Biswas, P. Macromolecules 2010, 43, 7378.
[31] Cui, W.; Su, C. F.; Merlitz, H.; Wu, C. X.; Sommer, J. U. Macromolecules 2014, 47, 3645.
[32] Bosko, J. T.; Prakash, J. R. Macromolecules 2011, 44, 660.
[33] Rubio, A. M.; McBride, C.; Freire, J. J. Macromolecules 2014, 47, 5379.
[34] Mandal, T.; Dasgupta, C.; Maiti, P. K. J. Chem. Phys. 2014, 141, 144901.
[35] Lu, Y.; An, L.; Wang, Z. G. Macromolecules 2013, 46, 5731.
[36] Jana, C.; Jayamurugan, G.; Ganapathy, R.; Maiti, P. K.; Jayaraman, N.; Sood, A. K. J. Chem. Phys. 2006, 124, 204719.
[37] Porcar, L.; Hong, K.; Butler, P. D.; Herwig, K. W.; Smith, G. S.; Liu, Y.; Chen, W. R. J. Phys. Chem. B 2010, 114, 1751.
[38] Prosa, T. J.; Bauer, B. J.; Amis, E. J. Macromolecules 2001, 34, 4897.
[39] Pötschke, D.; Ballauf, M.; Lindner, P.; Fischer, M.; Vögtle, F. J. Appl. Crystallogr. 2000, 33, 605.
[40] Rosenfeldt, S.; Dingenouts, N.; Ballauf, M.; Werner, N.; Vögtle, F.; Lindner, P. Macromolecules 2002, 35, 8098.
[41] Prosa, T. J.; Bauer, B. J.; Amis, E. J.; Tomalia, D. A.; Scherrenberg, R. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 2913.
[42] Rathgeber, S.; Monkenbusch, M.; Kreitschmann, M.; Urban, V.; Brulet, A. J. Chem. Phys. 2002, 117, 4047.
[43] Caminade, A. M.; Laurent, R.; Majoral, J. P. Adv. Drug Deliver. Rev. 2005, 57, 2130.
[44] Tanaka, H.; Koizumi, S.; Hashimoto, T.; Iyoh, H.; Satoh, M.; Naka, K.; Chujo, Y. Macromolecules 2007, 40, 4327.
[45] Klos, J. S.; Sommer, J. U. Macromolecules 2013, 46, 3107.
[46] Shi, M.; Yang, Y. Z.; Qiu, F. Acta Chim. Sinica 2018, 76, 715(in Chinese). (石梦, 杨颖梓, 邱枫, 化学学报, 2018, 76, 715)
[47] Mallamace, F.; Canetta, E.; Lombardo, D.; Mazzaglia, A.; Romeo, A.; Scolaro, L. M.; Maino, G. Physica A 2002, 304, 235.
[48] Yang, Y. Z.; Qiu, F.; Zhang, H. D.; Yang, Y. L. Macromolecules 2017, 50, 4007.
[49] Yang, Y. L.; Qiu, F.; Tang, P.; Zhang, H. D. Sci. China Chem. 2006, 36, 1(in Chinese). (杨玉良, 邱枫, 唐萍, 张红东, 中国科学B辑化学, 2006, 36, 1.)
/
| 〈 |
|
〉 |