

有水条件下路易斯酸B(C6F5)3对醛酮的选择性催化还原
收稿日期: 2018-10-11
网络出版日期: 2018-11-08
基金资助
项目受国家自然科学基金(No.21542011)和乐山师范学院科研项目(Z1414,Z1308)资助.
B(C6F5)3-Catalyzed Chemoselective Reduction of Carbonyl Compounds under Water Conditions
Received date: 2018-10-11
Online published: 2018-11-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 21542011), and Scientific Research Fund of Leshan Normal University (Z1414, Z1308).
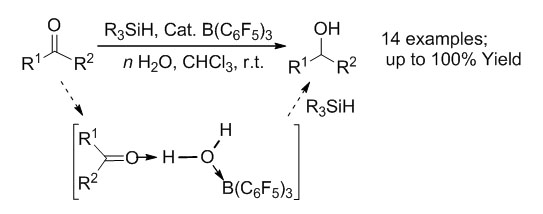
近年路易斯酸B(C6F5)3催化的醛酮还原反应研究表明,路易斯酸B(C6F5)3也可以作为一种"耐水"的催化剂在"有水"条件下进行催化反应.这些研究成果对进一步扩展受限路易斯酸碱对(FLPs)化学在水相中的应用及其发展至关重要.以硅烷作为还原剂,在路易斯酸B(C6F5)3催化下成功对14种不同取代的醛酮在温和条件下实现了高效地还原成醇反应,产率可高达100%.通过核磁共振分析对反应机理进行的研究表明,在有水条件下,底物羰基氧与催化剂硼烷之间存在以水介导的相互作用,即可能存在"R1R2C=O——H-O(-H)——B(C6F5)3"的三组分络合形式.对不同路易斯碱与水及硼烷所组成的"LB——H-O(-H)——B(C6F5)3"三组分复合物之间对应原子间距进行了比较研究,发现这种以水介导的相互作用对醛酮的羰基不仅具有质子化的活化作用,而且与硼烷的络合作用也更有利于硅烷作为还原剂在有水条件下使用.所报道的以硅烷作为还原剂,B(C6F5)3催化的醛酮直接还原成醇反应,首次实现了FLPs在真正有水条件下的催化反应,并更进一步证明了在以硅烷作为还原剂时,体系中存在的水不仅会参与催化反应并会促使反应的进行.对有水条件下FLPs催化的醛酮还原的选择性及其催化反应机理,以及对其他反应的影响还需要更深入的研究.
孙国峰 , 何云清 , 田冲 , Borzov Maxim , 胡启山 , 聂万丽 . 有水条件下路易斯酸B(C6F5)3对醛酮的选择性催化还原[J]. 化学学报, 2019 , 77(2) : 166 -171 . DOI: 10.6023/A18100423
Recently, the research work concerning B(C6F5)3 catalyzed reduction of carbonyl compounds revealed that this Lewis acid B(C6F5)3 presents, actually, a rather water-tolerant system. This fact considerably broadens the scope of the water/base tolerant frustrated Lewis pairs (FLP) chemistry. In this research, an efficient chemoselective reduction of aldehydes and ketones to alcohols catalyzed by the Lewis acid B(C6F5)3 has been developed. It is the first report about the chemoselective reduction of carbonyl compounds under aqueous conditions catalyzed by FLPs with hydridosilanes as reducing agents. The selectivity and activity of different hydridosilanes and the influence of substituents in carbonyl compounds have been studied. The effect of water concentration on the chemoselectivity of the reaction has also been investigated. It has been found that a 2~3 fold excess of water relatively to hydridosilanes usually exhibits better selectivity and overall yields than in the equimolar case. The reduction reaction can even be successfully performed with pure water as a solvent without any loss of the reactivity. Such a procedure has been successfully applied to reduce 14 differently substituted aldehydes and ketones into alcohols with up to 100% yields under mild conditions, but failed in case of the diaryl substituted ketones. Both experimental and computational methods have been performed to confirm the possibility of the water mediated mechanism and the effects of different Lewis bases on the LB——H-OH——LA three-component aggregates. These mechanistic studies have revealed that such water mediation between a carbonyl compound and a catalyst advantageously (i) activates the C=O group by protonation and (ii) fixes the catalytic borane moiety by formation of a B-O bond, which to some extent prevents the direct hydrolysis of hydridosilane and makes the reaction possible under moist conditions. Detailed clarification of the actual role of water in the reduction reaction of question will promote the further development of FLP-catalyzed and related reactions in the "green" chemistry field.

[1] de Vries, J. G.; Elsevier, C. J. The Handbook of Homogeneous Hydrogenation, Wiley-VCH, Weinheim, Germany, 2008.
[2] Sorella, G. L.; Sperni, L.; Canton, P.; Coletti, L.; Fabris, F.; Strukul, G.; Scarso, A. J. Org. Chem. 2018, 83(14), 7438.
[3] Leischner, T.; Spannenberg, A.; Junge, K.; Beller, M. Organometallics 2018, DOI:10.1021/acs.organomet.8b00410.
[4] Cao, Y.; Ma, R.; Wang, N.; Wang, M.-Y.; Li, X.-D.; He, L.-N. J. CO2 Util. 2018, 24, 328.
[5] Call, A.; Lloret-Fillol, J. Chem. Commun. 2018, 54, 9643.
[6] Zhang, J.; Qu, L.; Shi, G.; Liu, J.; Chen, J.; Dai, L. Angew. Chem., Int. Ed. 2016, 55, 2230.
[7] Chakraborty, S.; Bhattacharya, P.; Dai, H.; Guan, H. Acc. Chem. Res. 2015, 48, 1995.
[8] Guo, J.; Chen, J.; Lu, Z. Chem. Commun. 2015, 51, 5725.
[9] Dai, L.; Xue, Y.; Qu, L.; Choi, H. J.; Baek, J. B. Chem. Rev. 2015, 115, 4823.
[10] Mahdi, T.; Stephan, D. W. Angew. Chem., Int. Ed. 2015, 54, 8511.
[11] Volkov, A.; Gustafson, K. P. J.; Tai, C. W.; Verho, O.; Baeckvall, J. E.; Adolfsson, H. Angew. Chem., Int. Ed. 2015, 54, 5122.
[12] Parks, D. J.; Piers, W. E. J. Am. Chem. Soc. 1996, 118, 9440.
[13] Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2010, 49, 46.
[14] Liu, Y.-B.; Du, H.-F. Acta Chim. Sinica 2014, 72, 771(in Chinese). (刘勇兵, 杜海峰, 化学学报, 2014, 72, 771.)
[15] Xuan, Q.; Zhao, C.; Song, Q. Org. Biomol. Chem. 2017, 15, 5140.
[16] Wei, S.; Du, H. J. Am. Chem. Soc. 2014, 136, 12261.
[17] Oestreich, M.; Hermeke, J.; Mohr, J. Chem. Soc. Rev. 2015, 44, 2202.
[18] Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2015, 54, 6400.
[19] Ren, X.; Du, H. J. Am. Chem. Soc. 2016, 38, 810.
[20] Stephan, D. W.; Greenberg, S.; Graham, T. W.; Chase, P.; Hastie, J. J.; Geier, S. J.; Farrell, J. M.; Brown, C. C.; Heiden, Z. M.; Welch, G. C.; Ullrich, M. Inorg. Chem. 2011, 50, 12338.
[21] Mahdi, T. Stephan, D. W. J. Am. Chem. Soc. 2014, 136, 15809.
[22] Scott, D. J.; Fuchter, M. J.; Ashley, A. E. J. Am. Chem. Soc. 2014, 136, 15813.
[23] Scott, D. J.; Simmons, T. R.; Lawrence, E. J.; Wildgoose, G. G.; Fuchter, M. J.; Ashley, A. E. ACS Catal. 2015, 5, 5540.
[24] Gyömöre, A.; Bakos, M.; Földes, T.; Papai, I.; Domja, N. A.; Soós, T. ACS Catal. 2015, 5, 5366.
[25] Parks, D. J.; Blackwell, J. M.; Piers, W. E. J. Org. Chem. 2000, 65, 3090.
[26] Piers, W. E.; Marwitz, A. J. V.; Mercier, L. G. Inorg. Chem. 2011, 50, 12252.
[27] Nie, W.-L.; Klare, H. F. T.; Oestreich, M.; Froehlich, R.; Kehr, G.; Erker, G. Z. Naturforsch. 2012, 67b, 987.
[28] Ermeke, J.; Mewald, M.; Oestreich, M. J. Am. Chem. Soc. 2013, 135, 17537.
[29] Houghton, A. Y.; Hurmalainen, J.; Mansikkamaeki, A.; Piers, W. E.; Tuononen, H. M. Nat. Chem. 2014, 6, 983.
[30] Nimmagadda, R. D.; McRae, C. Tetrahedron Lett. 2006, 47, 5755.
[31] Chatterjee, I.; Porwal, D.; Oestreich, M. Angew. Chem., Int. Ed. 2017, 56, 3389.
[32] Yang, W.-Y.; Gao, L.; Lu, J.; Song, Z.-L. Chem. Commun. 2018, 54, 4834.
[33] Parks, D. J.; Piers, W. E. J. Am. Chem. Soc. 1996, 118, 9440.
[34] Blackwell, J. M.; Foster, K. L.; Beck, V. H.; Piers, W. E. J. Org. Chem. 1999, 64, 4887.
[35] Tahara, A.; Sunada, Y.; Takeshita, T.; Inoue, R.; Nagashima, H. Chem. Commun. 2018, 54, 11192.
[36] Hu, X.; Tian, C.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica, 2015, 73, 1025(in Chinese). (胡茜, 田冲, Borzov Maxim, 聂万丽, 化学学报, 2015, 73, 1025.)
[37] Tian, C.; Jiang, Y.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2015, 73, 1203(in Chinese). (田冲, 姜亚, Borzov Maxim, 聂万丽, 化学学报, 2015, 73, 1203).
[38] Wen, Z.-G.; Tian, C.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2016, 74, 498(in Chinese). (温志国, 田冲, Borzov Maxim, 聂万丽, 化学学报, 2016, 74, 498).
[39] Nie, W.-L.; Sun, G.-F.; Tian, C.; Borzov, M. Z. Naturforsch. 2016, 71(10)b, 1029.
[40] Zhang, L.-W.; Wen, Z.-G.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2017, 75, 819(in Chinese). (张露文, 温志国, Borzov Maxim, 聂万丽, 化学学报, 2017, 75, 819).
[41] Fasano, V.; Radcliffe, J. E.; Ingleson, M. J. ACS Catal. 2016, 6(3), 1793.
[42] Fasano, V.; Ingleson, M. J. Chem. Eur. J. 2017, 23(9), 2217.
[43] Sun, G.-F.; Su, M.; Fang, J.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2017, 75, 824(in Chinese). (孙国峰, 苏敏, 方洁, Borzov Maxim, 聂万丽, 化学学报, 2017, 75, 824).
[44] He, Y.-Q.; Teng, J. W.; Tian, C.; Borzov, M.; Hu, Q. S.; Nie, W.-L. Acta Chim. Sinica 2018, 76, 774(in Chinese). (何云清, 腾金伟, 田冲, Borzov Maxim, 胡启山, 聂万丽, 化学学报, 2018, 76, 774).
[45] He, Y.-Q.; Zou, M.-Y.; Xue, Y.; Hu, Q.-S.; Borzov, M. V.; Nie, W.-L. Mechanism Aspects of the B(C6F5)3 Catalyzed Reductive Amination, Chem-Eur. J. 2018, Submitted.
[46] Bergamaschi, G.; Lascialfari, L.; Pizzi, A.; Espinoza, M. I. M.; Demitri, N.; Milani, A.; Gori, A.; Metrangolo, P. Chem. Commun. 2018, DOI:10.1039/C8CC06010.
[47] Beck, A. D. J. Chem. Phys. 1993, 98, 5648.
[48] Parr, R. G.; Yang, W. Density Functional Theory of Atoms and Molecules, Oxford University Press, Oxford, 1989.
[49] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O. Nakai, H. Vreven, T. Throssell, K. Montgomery, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016.
[50] The typical 11B NMR signal of[R3NH] [HO-B(C6F5)3] is located at δ-3.9; the corresponding 19F NMR signals of o-, p-and m-F in[HO-B(C6F5)3] are at δ -135.6, -160.1, -164.8, respectively.
[51] Bergquist, C.; Bridgewater, B. M.; Harlan, C. J.; Norton, J. R.; Friesner, R. A.; Parkin, G. J. Am. Chem. Soc. 2000, 122, 10581.
[52] Di Saverio, A.; Focante, F.; Camurati, I.; Resconi, L.; Beringhelli, T.; D'Alfonso, G.; Donghi, D.; Maggioni, D.; Mercandelli, P.; Sironi, A. Inorg. Chem. 2005, 44, 5030.
/
| 〈 |
|
〉 |