

纳米零价铁在水相反应中的表面化学和晶相转化
收稿日期: 2018-10-02
网络出版日期: 2018-11-13
基金资助
项目受国家自然科学基金(Nos.41673096,41772243,51578398)和“博新计划”(BX201700172)资助.
Surface Chemistry and Phase Transformation of Nanoscale Zero-Valent Iron (nZVI) in Aquatic Media
Received date: 2018-10-02
Online published: 2018-11-13
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 41673096, 41772243, 51578398) and National Postdoctoral Program for Innovative Talents (BX201700172).
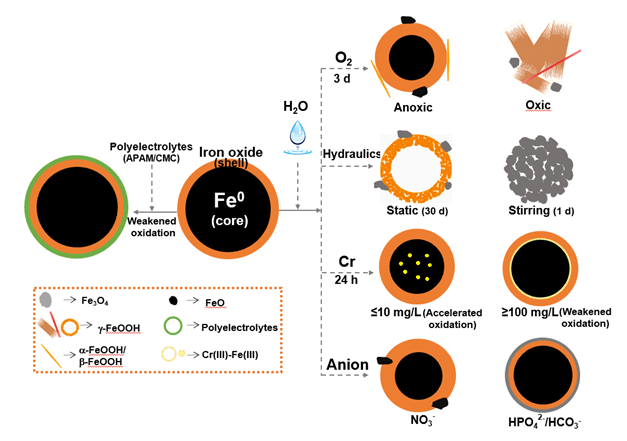
纳米零价铁在水相中的表面化学特性和晶相等性质变化,将影响其反应活性及环境归趋等.总结近期课题组关于纳米零价铁在水相中表面化学和晶相转化的研究进展,为纳米零价铁污染控制化学提供基础理论.重点探讨水中有无溶解氧、不同水力学条件复氧(静态和扰动)、重金属共存、无机阴离子共存对纳米零价铁颗粒表面化学特性和晶相转变的影响.同时也研究高分子电解质表面修饰后,颗粒在水相中表面及晶相的演变及对重金属去除性能的影响.研究表明,纳米零价铁与水相中的水分子、溶解氧、重金属离子及无机阴离子反应,零价铁失去电子演变为氧化铁、羟基氧化铁等;环境条件对颗粒结构性能产生影响,从而影响污染物去除效率及其在环境中的归趋.未来研究将重点探讨结构性能动态变化与不同污染物之间反应性能的影响,建立纳米颗粒的结构与性能之间关系模型,为纳米零价铁材料的环境应用提供理论依据.
刘静 , 刘静 , 顾天航 , 顾天航 , 王伟 , 王伟 , 刘爱荣 , 刘爱荣 , 张伟贤 , 张伟贤 . 纳米零价铁在水相反应中的表面化学和晶相转化[J]. 化学学报, 2019 , 77(2) : 121 -129 . DOI: 10.6023/A18100412
The unique "core-shell" structure endows nanoscale zero-valent iron (nZVI) rich aquatic surface chemistry properties. Transformation of surface chemistry and crystal phase of nZVI affect its reactivity and environmental transport and fate. Recent advances on the surface chemistry and phase transformation of nZVI in aqueous media are highlighted in this paper focusing on a basic theory of nZVI for pollution control and environmental application. Surface chemistry and phase of both fresh and reacted nZVI are presented. The structure, composition and properties of nanoparticles are determined not only by reaction time but also by environmental conditions. Specifically, the influences of dissolved oxygen, hydraulic conditions (static and stirring), types and concentrations of heavy metals (U(VI), Cr(VI), Se(IV)) and anions (NO3-, SO42-, HPO42- and HCO3-) are investigated. In addition, the effect of surface modification with polyelectrolytes, including anionic polyacrylamide (APAM) and carboxymethylcellulose sodium (CMC), on microstructure, morphology and composition of nanoparticles in aqueous phase was discussed. Results demonstrate that environmental conditions have significant impacts on particles structure, composition and properties, consequently on nZVI performance for pollutant removal. After corrosion under different aqueous conditions, the core-shell structured nZVI are distorted and the metallic iron core is transformed into different iron oxides/hydroxides, such as γ-Fe2O3, Fe3O4 and γ-FeOOH. These iron (hydr)oxides exhibit different surface complexation and affinity proprieties, thus eventually affecting the pollutant removal performance and the environmental fate of reaction products. More research on the effect of dynamic structure transformation by different types of pollutants, and a reaction model between the surface chemistry/phase transformation and removal performance are needed to deepen our understanding on nZVI surface chemistry, and develop more effective technologies of environmental applications.

[1] Alowitz, M. J.; Scherer, M. M. Environ. Sci. Technol. 2002, 36, 299.
[2] Johnson, T. L.; Scherer, M. M.; Tratnyek, P. G. Environ. Sci. Technol. 1996, 30, 2634.
[3] Matheson, L. J.; Tratnyek, P. G. Environ. Sci. Technol. 1994, 28, 2045.
[4] Bang, S.; Korfiatis, G. P.; Meng, X. J. Hazard. Mater. 2005, 121, 61.
[5] Wang, C. B.; Zhang, W. X. Environ. Sci. Technol. 1997, 31, 2154.
[6] Shu, H. Y.; Chang, M. C.; Chen, C. C.; Chen, P. E. J. Hazard. Mater. 2010, 184, 499.
[7] Sohn, K.; Kang, S. W.; Ahn, S.; Woo, M.; Yang, S. K. Environ. Sci. Technol. 2006, 40, 5514.
[8] Huang, X. Y.; Wang, W.; Ling, L.; Zhang, W. X. Acta Chim. Sinica 2017, 75, 529(in Chinese). (黄潇月, 王伟, 凌岚, 张伟贤, 化学学报, 2017, 75, 529.)
[9] Gu, T.; Shi, J.; Hua, Y.; Liu, J.; Wang, W.; Zhang, W. X. Acta Chim. Sinica 2017, 75, 991(in Chinese). (顾天航, 石君明, 滑熠龙, 刘静, 王伟, 张伟贤, 化学学报, 2017, 75, 991.)
[10] Xia, X.; Hua, Y.; Huang, X.; Ling, L.; Zhang, W. X. Acta Chim. Sinica 2017, 75, 594(in Chinese). (夏雪芬, 滑熠龙, 黄潇月, 凌岚, 张伟贤, 化学学报, 2017, 75, 594.)
[11] Liu, A.; Liu, J.; Han, J.; Zhang, W. X. J. Hazard. Mater. 2017, 322, 129.
[12] Liu, A. R.; Liu, J.; Zhang, W. X. Chemosphere 2015, 119, 1068.
[13] Liu, A. R.; Liu, J.; Pan, B. C.; Zhang, W. X. RSC Adv. 2014, 4, 57377.
[14] Mu, Y.; Jia, F.; Ai, Z.; Zhang, L. Acta Chim. Sinica 2017, 75, 538 (in Chinese). (穆毅, 贾法龙, 艾智慧, 张礼知, 化学学报, 2017, 75, 538.)
[15] Wang, C. Y.; Chen, Z. Y.; Cheng, B.; Zhu, Y. R.; Liu, H. J. Mater. Sci. Eng. B 1999, 60, 223.
[16] Liu, A. R.; Zhang, W. X. Analyst 2014, 139, 4512.
[17] Wang, C.; Baer, D. R.; Amonette, J. E.; Engelhard, M. H.; Antony, J.; Qiang, Y. J. Am. Chem. Soc. 2009, 131, 8824.
[18] Nurmi, J. T.; Tratnyek, P. G.; Sarathy, V.; Baer, D. R.; Amonette, J. E.; Pecher, K.; Wang, C.; Linehan, J. C.; Matson, D. W.; Penn, R. L. Environ. Sci. Technol. 2005, 39, 1221.
[19] Yan, W.; Herzing, A. A.; Kiely, C. J.; Zhang, W. X. J. Contam. Hydrol. 2010, 118, 96.
[20] Liu, Y.; Majetich, S. A.; Tilton, R. D.; Sholl, D. S.; Lowry, G. V. Environ. Sci. Technol. 2005, 39, 1338.
[21] Kim, H. S.; Kim, T.; Ahn, J. Y.; Hwang, K. Y.; Park, J. Y.; Lim, T. T.; Hwang, I. Chem. Eng. J. 2012, 197, 16.
[22] Kanel, S. R.; Greneche, J. M.; Choi, H. Environ. Sci. Technol. 2006, 40, 2045.
[23] Ling, L.; Zhang, W. X. Environ. Sci. Technol. Lett. 2014, 1, 209.
[24] Yan, W. L.; Herzing, A. A.; Li, X. Q.; Kiely, C. J.; Zhang, W. X. Environ. Sci. Technol. 2010, 44, 4288.
[25] Ling, L.; Zhang, W. X. RSC Adv. 2014, 4, 33861.
[26] Ling, L.; Huang, X. Y.; Zhang, W. X. Adv. Mater. 2018, 1705703.
[27] Ling, L.; Zhang, W. X. Environ. Sci. Technol. Lett. 2014, 1, 305.
[28] Kanel, S. R.; Manning, B.; Charlet, L.; Choi, H. Environ. Sci. Technol. 2005, 39, 1291.
[29] Feng, H.; Tang, L.; Tang, J.; Zeng, G.; Dong, H.; Deng, Y.; Wang, L.; Liu, Y.; Ren, X.; Zhou, Y. Environ. Sci. Nano 2018, 5, 1595.
[30] Tang, L.; Feng, H.; Tang, J.; Zeng, G.; Deng, Y.; Wang, J.; Liu, Y.; Zhou, Y. Water Res. 2017, 117, 175.
[31] Ling, L.; Pan, B. C.; Zhang, W. X. Water Res. 2015, 71, 274.
[32] Ling, L.; Zhang, W. X. J. Am. Chem. Soc. 2015, 137, 2788.
[33] Huang, X. Y.; Ling, L.; Zhang, W. X. J. Environ. Sci. 2018, 67, 4.
[34] Xia, X. F.; Ling, L.; Zhang, W. X.. Environ. Sci. Nano 2017, 4, 52.
[35] Xia, X. F.; Ling, L.; Zhang, W. X. Chemosphere 2017, 168, 1597.
[36] Liu, H. B.; Chen, T. H.; Chang, D. Y.; Chen, D.; Liu, Y.; He, H. P.; Yuan, P.; Frost, R. Mater. Chem. Phys. 2012, 133, 205.
[37] Liu, Y.; Phenrat, T.; Lowry, G. V. Environ. Sci. Technol. 2007, 41, 7881.
[38] Reinsch, B. C.; Forsberg, B.; Penn, R. L.; Kim, C. S.; Lowry, G. V. Environ. Sci. Technol. 2010, 44, 3455.
[39] Almeelbi, T.; Bezbaruah, A. J. Nanopart. Res. 2012, 14, 900.
[40] Wen, Z.; Zhang, Y.; Dai, C. Colloid. Surface A 2014, 457, 433.
[41] Hua, Y.; Wang, W.; Huang, X.; Gu, T.; Ding, D.; Ling, L.; Zhang, W. X. Chemosphere 2018, 201, 603.
[42] Jiemvarangkul, P.; Zhang, W. X.; Lien, H. L. Chem. Eng. J. 2011, 170, 482.
[43] Johnson, R. L.; Nurmi, J. T.; O'Brien Johnson, G. S.; Fan, D.; O'Brien Johnson, R. L.; Shi, Z.; Salter-Blanc, A. J.; Tratnyek, P. G.; Lowry, G. V. Environ. Sci. Technol. 2013, 47, 1573.
[44] Liu, J.; Liu, A. R.; Zhang, W. X. Chem. Eng. J. 2016, 303, 26832.
[45] Tang, J.; Tang, L.; Feng, H.; Dong, H.; Zhang, Y.; Liu, S.; Zeng, G. Acta Chim. Sinica 2017, 75, 575(in Chinese). (汤晶, 汤琳, 冯浩朋, 董浩然, 章毅, 刘思诗, 曾光明, 化学学报, 2017, 75, 575.)
[46] Su, Y. M.; Adeleye, A. S.; Keller, A. A.; Huang, Y. X.; Dai, C. M.; Zhou, X. F.; Zhang, Y. L. Water Res. 2015, 74, 47.
[47] Rajajayavel, S. R. C.; Ghoshal, S. Water Res. 2015, 78, 144.
/
| 〈 |
|
〉 |