

氢氟酸盐和三价碘实现的β,γ-不饱和羧酸脱羧氟化反应
收稿日期: 2018-08-07
网络出版日期: 2018-11-19
基金资助
项目受国家自然科学基金(Nos.21776138,21476116),中央高校基本科研专项资金(Nos.30916011102,30918011314),江苏省自然科学基金(BK20180476),江苏高校优势学科建设工程项目,青蓝工程和六大人才高峰对本项目的资金支持.
Fluorodecarboxylation of β,γ-Unsaturated Carboxylic Acids Using Trivalent Iodine and Hydrofluoric Acid-Based Fluorination Reagent
Received date: 2018-08-07
Online published: 2018-11-19
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21776138, 21476116), Fundamental Research Funds for the Central Universities (Nos. 30916011102, 30918011314), Natural Science Foundation of Jiangsu Province (BK20180476), Qing Lan and Six Talent Peaks in Jiangsu Province, Priority Academic Program Development of Jiangsu Higher Education Institutions.
宋治东 , 蒋绿齐 , 易文斌 . 氢氟酸盐和三价碘实现的β,γ-不饱和羧酸脱羧氟化反应[J]. 化学学报, 2018 , 76(12) : 967 -971 . DOI: 10.6023/A18080321
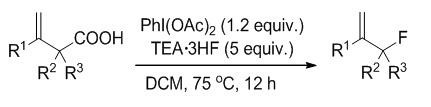
Allylic-substituted compounds serve as versatile building block or the primer for many metal-catalyzed reactions. The introduction of fluorine into a drug molecule will change its pharmacokinetic and pharmacodynamic properties. Therefore, a new method of allylic fluorination would uncover novel synthetic approaches towards highly valuable fluorinated compounds such as inhibitors or fluorine-containing polypropylene. To date, the most reported methods for the synthesis of allylic fluoride involve the use of p-nitrobenzoate or trimethylsilyl as leaving group, or cleavage of tertiary cyclopropyl silyl ethers. In the past decades, the research of fluorodecarboxylation has made great progress. The most reported fluorodecarboxylations involving XeF2, AgF or AgF2, Selectfluor, N-fluorodibenzenesulfonimide (NFSI) are often accompanied by the occurrence of oxidation or free radical reactions, which may destroy the terminal olefin structure. The use of the fluoride ion (fluoride salt or hydrofluoric acid) as the nucleophilic component presents a series of challenges, including the low intrinsic nucleophilicity which was demonstrated by its frequent use as an additive to modulate catalytic reactivity or product distribution. However, with the assistance of transition-metal catalyst or organocatalysts, fluoride ion often serve as fluorine source for fluorination of C(sp2)―H and C(sp3)―H. Hypervalent iodine reagents, which have ability to activate a C―C multiple bond, have been recognized as an alternative to noble metal catalyst. Inspired by the pioneering exploration, we sought the possibility of achieving allylic fluorination through simple protocol of fluorodecarboxylation with cheap nucleophilic fluorination reagents and mild oxidant. In this work, a new strategy is introduced for the synthesis of allylic fluorides via decarboxylative fluorination of β,γ-unsaturated carboxylic acids using PhI(OAc)2 and TEA·3HF. The best result was achieved by using 1.2 equiv. of PhI(OAc)2 and 5 equiv. of TEA·3HF in CH2Cl2 at 75 ℃ for 12 h, giving allylic fluoride 2a in 76% yield. The versatile synthetic utilities of the allylic fluorides were also developed through cycloaddition, oxidation, reduction, substitution involving formation of C―O, C―S, C―Se and C―N bond via activation of C―F bond.
[1] (a) Singh, O. V.; Han, H. Org. Lett. 2007, 9, 4801;
(b) Mizuno, S.; Terasaki, S.; Shinozawa, T.; Kawatsura, M. Org. Lett. 2017, 19, 504.
(c) Wu, R. H.; Yang, W.; Cheng, G.; Li, Y.; Yang, D. Q. Chinese J. Org. Chem. 2016, 36, 2368(in Chinese). (仵瑞华, 杨文, 程果, 李玥, 杨定乔, 有机化学, 2016, 36, 2368);
(d) Chen, C. H.; Fu, L.; Chen, P. H.; Liu, G. S. Chin. J. Chem. 2017, 35, 1781;
(e) Gu, Y.; Lu, C.; Gu, Y.; Shen, Q. Chin. J. Chem. 2018, 36, 55.
[2] (a) Kirsch, P. Modern Fluoroorganic Chemistry:Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, Germany, 2004;
(b) Hiyama, T. Organofluorine Compounds:Chemistry and Applications, Springer, Berlin, 2000;
(c) Uneyama, K. Organofluorine Chemistry, Blackwell, Oxford, U.K., 2006;
(d) Richard, C. Fluorine in Organic Chemistry, CRC Press, Boca Raton, FL, 2004.
[3] (a) Honda, T.; Liby, K. T.; Su, X.; Sundararajan, C.; Honda, Y.; Suh, N.; Risingsong, R.; Williams, C. R.; Royce, D. B.; Sporn, M. B.; Gribble, G. W. Bioorg. Med. Chem. Lett. 2006, 16, 6306;
(b) Kaneko, S.; Arai, M.; Uchida, T.; Harasaki, T.; Fukuoka, T.; Konosu, T. Bioorg. Med. Chem. Lett. 2002, 12, 1705;
(c) Rothman, S. C.; Johnston, J. B.; Lee, S.; Walker, J. R.; Poulter, C. D. J. Am. Chem. Soc. 2008, 130, 4906;
(d) Leblanc, Y.; Roy, P.; Leger, S.; Grimm, E.; Wang, Z. WO 9841516, 1998[Chem. Abstr. 1998, 129, 275831].
[4] (a) Walkowiak-Kulikowska, J.; Szwajca, A.; Boschet, F.; Gouverneur, V.; Ameduri, B. Macromolecules 2014, 47, 8634.
(b) Kostov, G.; Tredwell, M.; Gouverneur, V.; Ameduri, B. J. Polym. Sci., Part A:Polym. Chem. 2007, 45, 3843.
(c) Wall, L. A. Fluoropolymers, Wiley, New York, 1972.
[5] Hollingworth, C.; Hazari, A.; Hopkinson, M. N.; Tredwell, M.; Benedetto, E.; Huiban, M.; Gee, A. D.; Brown, J. M.; Gouverneur, V. Angew. Chem., Int. Ed. 2011, 50, 2613.
[6] Walkowiak, J.; Martinez, D. C. T.; Ameduri, B.; Gouverneur, V. Synthesis 2010, 1883.
[7] (a) Kirihara, M.; Kambayashi, T.; Momose, T. Chem. Commun. 1996, 1103;
(b) Kirihara, M.; Kakuda, H.; Tsunooka, M.; Shimajiri, A.; Takuwa, T.; Hatano, A. Tetrahedron Lett. 2003, 44, 8513.
[8] Hu, J. B.; He, Z. B. CN 102219638, 2011[Chem. Abstr. 2011, 155, 588760].
[9] (a) He, Z. B.; Ping, T.; Hu, J. B. Org. Lett. 2016, 18, 72;
(b) Ma, J. J.; Yi, W. B.; Lu, G. P.; Cai, C. Adv. Synth. Catal. 2015, 357, 3447.
(c) Xu, X. L.; Chen, H. H.; He, J. B.; Xu, H. J. Chin. J. Chem. 2017, 35, 1665;
(d) Montazerozohori, M.; Nasr-Esfahani, M.; Akhlaghi, P. Chin. J. Chem. 2009, 27, 1007;
(e) Zhang, J. J.; Cheng, Y. B.; Duan, X. H. Chin. J. Chem. 2017, 35, 311;
(f) Zhao, Y. W.; Feng, Q.; Song, Q. L. Chin. Chem. Lett. 2016, 27, 571.
[10] (a) Chatalova-Sazepin, C.; Binayeva, M.; Epifanov, M.; Zhang, W.; Foth, P.; Amador, C.; Jagdeo, M.; Boswell, B. R.; Sammis, G. M. Org. Lett. 2016, 18, 4570;
(b) Patrick, T. B.; Khazaeli, S.; Nadji, S.; Hering-Smith, K.; Reif, D. J. Org. Chem. 1993, 58, 705.
[11] (a) Yin, F.; Wang, Z.; Li, Z.; Li, C. J. Am. Chem. Soc. 2012, 134, 10401;
(b) Zhang, X. Comput. Theor. Chem. 2016, 1082, 11;
(c) Zhang, Q. W.; Brusoe, A. T.; Mascitti, V.; Hesp, K. D.; Blakemore, D. C.; Kohrt, J. T.; Hartwig, J. F. Angew. Chem., Int. Ed. 2016, 55, 9758;
(d) Mizuta, S.; Stenhagen, I. S. R.; O'Duill, M.; Wolstenhulme, J.; Kirjavainen, A. K.; Forsback, S. J.; Tredwell, M.; Sandford, G.; Moore, P. R.; Huiban, M.; Luthra, S. K.; Passchier, J.; Solin, O.; Gouverneur, V. Org. Lett. 2013, 15, 2648.
[12] (a) Leung, J. C. T.; Chatalova-Sazepin, C.; West, J. G.; Rueda-Becerril, M.; Paquin, J. F.; Sammis, G. M. Angew. Chem., Int. Ed. 2012, 51, 10804;
(b) Leung, J. C. T.; Sammis, G. M. Eur. J. Org. Chem. 2015, 2197;
(c) Ventre, S.; Petronijevic, F. R.; MacMillan, D. W. C. J. Am. Chem. Soc. 2015, 137, 5654;
(d) Wang, D. H.; Yuan, Z. L.; Liu, Q. L.; Chen, P. H. Liu, G. S. Chin. J. Chem. 2018, 36, 507;
(e) Dong, Y.; Wang, Z.; Li, C. Nat. Commun. 2017, 8, 277.
[13] Rueda-Becerril, M.; Chatalova Sazepin, C.; Leung, J. C. T.; Okbinoglu, T.; Kennepohl, P.; Paquin, J. F.; Sammis, G. M. J. Am. Chem. Soc. 2012, 134, 4026.
[14] Yang, Q.; Mao, L. L.; Yang, B.; Yang, S. D. Org. Lett. 2014, 16, 3460.
[15] (a) Fagnou, K.; Lautens, M. Angew. Chem. 2002, 114, 26;
(b) Yan, N.; Lei, Z. W.; Su, J. K.; Liao, W. L. Hu, X. G. Chin. Chem. Lett. 2017, 28, 467;
(c) Wang, L. Y.; Jiang, X. H.; Tang, P. P. Org. Chem. Front. 2017, 4, 1958.
[16] (a) Katcher, M. H.; Sha, A.; Doyle, A. G. J. Am. Chem. Soc. 2011, 133, 15902;
(b) Lee, E.; Hooker, J. M.; Ritter, T. J. Am. Chem. Soc. 2012, 134, 17456;
(c) Fier, P. S.; Luo, J.; Hartwig, J. F. J. Am. Chem. Soc. 2013, 135, 2552;
(d) Fier, P. S.; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 10795;
(e) Liu, Z.; Chen, H.; Lv, Y.; Tan, X.; Shen, H.; Yu, H.-Z.; Li, C. J. Am. Chem. Soc. 2018, 140, 6169;
(f) Ma, J. A.; Li, S. Org. Chem. Front. 2014, 1, 712.
[17] Woerly, E. M.; Banik, S. M.; Jacobsen, E. N. J. Am. Chem. Soc. 2016, 138, 13858.
[18] Huang, X.; Liu, W.; Hooker, J. M.; Groves, J. T. Angew. Chem., Int. Ed. 2015, 54, 5241.
[19] (a) Souto, J. A.; Becker, P.; Iglesias, A.; Muñiz, K. J. Am. Chem. Soc. 2012, 134, 15505;
(b) Souto, J. A.; Martínez, C.; Velilla, I.; Muñiz, K. Angew. Chem., Int. Ed. 2013, 52, 324;
(c) Röben, C.; Souto, J. A.; Escudero-Adán, E. C.; Muñiz, K. Org. Lett. 2013, 15, 1008;
(d) Farid, U.; Malmedy, F.; Claveau, R.; Albers, L.; Wirth, T. Angew. Chem., Int. Ed. 2013, 52, 7018;
(e) Wang, Y.; Wang, Y.; Zhang, Q.; Li, D. Org. Chem. Front. 2017, 4, 514;
(f) Gao, P.; Fan, M. J.; Bai, Z. J.; Wei, Y. Y. Chin. J. Chem. 2015, 33, 479.
[20] (a) Kiyokawa, K.; Yahata, S.; Kojima, T.; Minakata, S. Org. Lett. 2014, 16, 4646;
(b) Kiyokawa, K.; Kojima, T.; Hishikawa, Y.; Minakata, S. Chem. Eur. J. 2015, 21, 15548.
[21] (a) Jiang, L. Q.; Qian, J. L.; Yi, W. B.; Lu, G. P.; Cai, C.; Zhang, W. Angew. Chem., Int. Ed. 2015, 54, 14965;
(b) Lin, Y.-M.; Yi, W. B.; Shen, W. Z.; Lu, G. P. Org. Lett. 2016, 18, 592;
(c) Song, Z. D.; Yi, W. B. Adv. Synth. Catal. 2016, 358, 2727.
[22] (a) Kitamura, T.; Muta, K.; Kuriki, S. Tetrahedron Lett. 2013, 54, 6118;
(b) Carpenter, W. J. Org. Chem. 1966, 31, 2688;
(c) Zupan, M.; Pollak, A. J. Fluorine Chem. 1976, 7, 445;
(d) Arrica, M. A.; Wirth, T. Eur. J. Org. Chem. 2005, 395;
(e) Ye, C.; Twamley, B.; Shreeve, J. M. Org. Lett. 2005, 7, 3961.
[23] Kitamura, T.; Muta, K.; Oyamada, J. J. Org. Chem. 2015, 80, 10431.
[24] Nash, T. J.; Pattison, G. Eur. J. Org. Chem. 2015, 3779.
[25] Li, Y.; Ni, C.; Liu, J.; Zhang, L.; Zheng, J.; Zhu, L.; Hu, J. B. Org. Lett. 2006, 8, 1693.
[26] (a) Fukuzumi, T.; Shibata, N.; Sugiura, M.; Yasui, H.; Nakamura, S.; Toru, T. Angew. Chem., Int. Ed. 2006, 45, 4973;
(b) Furukawa, T.; Shibata, N.; Mizuta, S.; Nakamura, S.; Toru, T.; Shiro, M. Angew. Chem., Int. Ed. 2008, 47, 8051.
[27] Prakash, G. K. S.; Ledneczki, I.; Chacko, S.; Olah, G. A. Org. Lett. 2008, 10, 557.
[28] Traff, A. M.; Janjetovic, M.; Ta, L.; Hilmersson, G. Angew. Chem., Int. Ed. 2013, 52, 12073.
[29] (a) Yi, W. B.; Huang, X.; Zhang, Z.; Zhu, D.; Cai, C.; Zhang, W. Green Chem. 2012, 14, 3185;
(b) Qian, J. L.; Yi, W. B.; Huang, X.; Miao, Y. B.; Zhang, J. K.; Cai, C.; Zhang, W. Org. Lett. 2015, 17, 1090;
(c) Song, Z. D.; Huang, X.; Yi, W. B.; Zhang, W. Org. Lett. 2016, 18, 5640.
[30] Benedetto, E.; Keita, M.; Tredwell, M.; Hollingworth, C.; Brown, J. M.; Gouverneur, V. Organometallics 2012, 31, 1408.
/
| 〈 |
|
〉 |