

收稿日期: 2018-11-21
网络出版日期: 2019-01-28
基金资助
项目受国家自然科学基金(Nos.21772066,21572229)以及广东省"特支"计划(No.2017TX04R059)资助.
Recent Advances of Chiral Hypervalent Iodine Reagents
Received date: 2018-11-21
Online published: 2019-01-28
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21772066, 21572229) and Guangdong Special Support Program (No. 2017TX04R059).
蔡倩 , 马浩文 . 手性高价碘试剂的发展及展望[J]. 化学学报, 2019 , 77(3) : 213 -230 . DOI: 10.6023/A18110470
Hypervalent iodine chemistry has arose as an important field in organic chemistry in the past decades. Hypervalent iodine compounds, with reactivities similarly to transition metals in many different types of transformations, have attracted broad interests in organic community due to their practical advantages in the mild conditions, low costs, environmental benign and low toxicity. Great progresses have been made in this field. Chiral hypervalent iodine reagents or precursors have also been developed and utilized in a variety of asymmetric reactions in a stoichiometric or catalytic way. Important advances have been witnessed in the field of chiral hypervalent iodine chemistry in recent years. However, great limitations still exist. In this review, we have made a summary of different types of chiral hypervalent iodine reagents and precursors according to the characteristics of these compounds and the timeline. It may be helpful for the researchers to better understand the development and limitations of chiral hypervalent iodine chemistry.
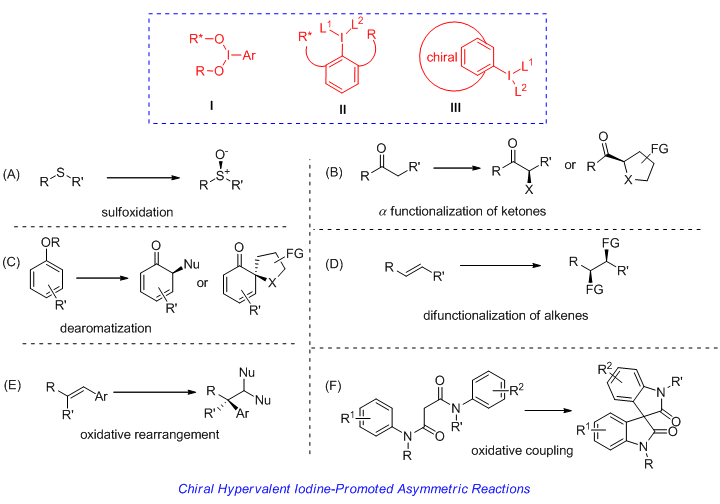
[1] For books, see:(a) Chemistry of Hypervalent Compounds, Ed.:AKiba, K. Y., Wiley-VCH, New York, 1999.
(b) Zhdankin, V. V. Hypervalent Iodine Chemistry:Preparation, Structure and Syn-thetic Application of Polyvalent Iodine Compounds, John Wiley & Sons Ltd., New York, 2014.
(c) Iodine Chemistry And Applications, Ed.:Kaiho, T., John Wiley & Sons Ltd., New York, 2015.
(d) Hypervalent Iodine Chemistry:Modern Developments in Organic Synthesis, Ed.:Wirth, T., Springer, 2003.
[2] For recent reviews, see:(a) Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328.
(b) Duan, Y.; Jiang, S.; Han, Y.; Sun, B.; Zhang, C. Chin. J. Org. Chem. 2016, 36, 1973(in Chinese). (段亚南, 姜山, 韩永超, 孙博, 张弛, 有机化学, 2016, 36, 1973.)
(c) Ma, J.; Chen, L.; Yuan, Z.; Cheng, H. Chin. J. Org. Chem. 2018, 38, 1586(in Chinese). (马姣丽, 陈立成, 袁中文, 程辉成, 有机化学, 2018, 38, 1586.)
[3] For selected recent reviews, see:(a) Flores, A.; Cots, E.; Bergès, J.; Muñiz, K. Adv. Synth. Catal. 2019, 361, DOI:10.1002/adsc. 201800521.
(b) Martín Romero, R.; Wöste, T. H.; Muñiz, K. Chem. Asian J. 2014, 9, 972.
(c) Singh, F. V.; Wirth, T. Chem. Asian J. 2014, 9, 950.
(d) Harned, A. M. Tetrahedron Lett. 2014, 55, 4681.
(e) Parra, A.; Reboredo, S. Chem. Eur. J. 2013, 19, 17244.
[4] Liang, H.; Ciufolini, M. A. Angew. Chem. Int. Ed. 2011, 50, 11849.
[5] Ochiai, M.; Takeuchi, Y.; Katayama, T.; Sueda, T.; Miyamoto, K. J. Am. Chem. Soc. 2005, 127, 12244.
(b) Dohi, T.; Maruyama, A.; Yoshimura, M.; Morimoto, K.; Tohma, H.; Kita, Y. Angew. Chem. Int. Ed. 2005, 44, 6193.
[6] Pribram, R. Justus Liebigs Ann. Chem. 1907, 351, 481.
[7] Imamoto, T.; Koto, H. Chem. Lett. 1986, 967.
[8] Hatzigrigoriou, E.; Varvoglis, A.; Bakola-Christianopoulou, M. J. Org. Chem. 1990, 55, 315.
[9] Xia, M.; Chen, Z.-C. Synth. Commun. 1997, 27, 1321.
[10] Ray Ⅲ, D. G.; Koser, G. F. J. Am. Chem. Soc. 1990, 112, 5672.
[11] Ray Ⅲ D. G.; Koser, G. F. J. Org. Chem. 1992, 57, 1607.
[12] Tohma, H.; Takizawa, S.; Watanabe, H.; Fukuoka, Y.; Maegawa, T.; Kita, Y. J. Org. Chem. 1999, 64, 3519.
[13] Rabah, G. A.; Koser, G. F. Tetrahedron Lett. 1996, 37, 6453.
[14] (a) Wirth, T.; Hirt, U. H. Tetrahedron Asymmetry 1997, 8, 23.
(b) Hirt, U. H.; Spingler, B.; Wirth, T. J. Org. Chem. 1998, 63, 7674.
(c) Hirt, U. H.; Schuster, M. F. H.; French, A. N.; Wiest, O. G.; Wirth, T. Eur. J. Org. Chem. 2001, 1569.
[15] Mizar, P.; Laverny, A.; EI-Sherbini, M.; Farid, U.; Brown, M.; Malmedy, F.; Wirth, T. Chem. Eur. J. 2014, 20, 9910.
[16] Hempel, C.; Maichle-Mössmer, C.; Pericàs, M. A.; Nachtsheim, B. J. Adv. Synth. Catal. 2017, 359, 2941.
[17] Fujita, M.; Okuno, S.; Lee, H. J.; Sugimura, T.; Okuyama, T. Tetrahedron Lett. 2007, 48, 8691.
[18] (a) Uyanik, M.; Yasui, T.; Ishihara, K. Angew. Chem. Int. Ed. 2010, 49, 2175.
(b) Uyanik, M.; Yasui, T.; Ishihara, K. Tetrahedron 2010, 66, 5841.
[19] (a) Fujita, M.; Yoshida, Y.; Miyata, K.; Wakisaka, A.; Sugimura, T. Angew. Chem. Int. Ed. 2010, 49, 7068.
(b) Fujita, M.; Mori, K.; Shimogaki, M.; Sugimura, T. Org. Lett. 2012, 14, 1294.
(c) Shimogaki, M.; Fujita, M.; Sugimura, T. Eur. J. Org. Chem. 2013, 7128.
(d) Takesue, T.; Fujita, M.; Sugimura, T.; Akutsu, H. Org. Lett. 2014, 16, 4634.
[20] Fujita, M.; Wakita, M.; Sugimura, T. Chem. Commun. 2011, 47, 3983.
[21] (a) Shimogaki, M.; Fujita, M.; Sugimura, T. Angew. Chem. Int. Ed. 2016, 55, 15797.
(b) Shimogaki, M.; Fujita, M.; Sugimura, T. J. Org. Chem. 2017, 82, 11836.
[22] Röben, C.; Souto, J. A.; González, Y.; Lishchynskyi, A.; Muñiz, K. Angew. Chem. Int. Ed. 2011, 50, 9478.
[23] Muñiz, K.; Barreiro, L.; Romero, R. M.; Martínez, C. J. Am. Chem. Soc. 2017, 139, 4354.
[24] (a) Haubenreisser, S.; Wöste, T. H.; Martínez, C.; Ishihara, K.; Muñiz, K. Angew. Chem. Int. Ed. 2016, 55, 413.
(b) Wöste, T. H.; Muñiz, K. Synthesis 2016, 48, 816.
[25] (a) Farid, U.; Wirth, T. Angew. Chem. Int. Ed. 2012, 51, 3462.
(b) Mizar, P.; Niebuhr, R.; Hutchings, M.; Farooq, U.; Wirth, T. Chem. Eur. J. 2016, 22, 1614.
[26] Gelis, C.; Dumoulin, A.; Bekkaye, M.; Neuville, L.; Masson, G. Org. Lett. 2017, 19, 278.
[27] (a) Kong, W.; Feige, P.; de Haro, T.; Nevado, C. Angew. Chem. Int. Ed. 2013, 52, 2469.
(b) Pluta, R.; Krach, P. E.; Cavallo, L.; Falivene, L.; Rueping, M. ACS Catal. 2018, 8, 2582.
[28] Wu, H.; He, Y.-P.; Xu, L.; Zhang, D.-Y.; Gong, L.-Z. Angew. Chem. Int. Ed. 2014, 53, 3466.
[29] Zhang, D.-Y.; Xu, L.; Wu, H.; Gong, L.-Z. Chem. Eur. J. 2015, 21, 10314.
[30] Cao, Y.; Zhang, X.; Lin, G.; Zhang-Negrerie, D.; Du, Y. Org. Lett. 2016, 18, 5580.
[31] Farid, U.; Malmedy, F.; Claveau, R.; Albers, C.; Wirth, T. Angew. Chem. Int. Ed. 2013, 52, 7018.
[32] Brown, M.; Kumar, R.; Rehbein, J.; Wirth, T. Chem. Eur. J. 2016, 22, 4030.
[33] Banik, S. M.; Medley, J. W.; Jacobsen, E. N. J. Am. Chem. Soc. 2016, 138, 5000.
[34] Banik, S. M.; Medley, J. W.; Jacobsen, E. N. Science 2016, 353, 51.
[35] Zhou, B.; Haj, M. K.; Jacobsen, E. N.; Houk, K. N.; Xue, X.-S. J. Am. Chem. Soc. 2018, 140, 15206.
[36] Mennie, K. M.; Banik, S. M.; Reichert, E. C.; Jacobsen, E. N. J. Am. Chem. Soc. 2018, 140, 4797.
[37] Qurban, J.; Elsherbini, M.; Wirth, T. J. Org. Chem. 2017, 82, 11872.
[38] Hashimoto, T.; Shimazaki, Y.; Omatsu, Y.; Maruoka, K. Angew. Chem. Int. Ed. 2018, 57, 7200.
[39] Zhdandin, V. V.; Smart, J. T.; Zhao, P.; Kiprof, P. Tetrahedron Lett. 2000, 41, 5299.
[40] Ladziata, U.; Carlson, J.; Zhdankin, V. V. Tetrahedron Lett. 2006, 47, 6301.
[41] Altermann, S. M.; Richardson, R. D.; Page, T. K.; Schmidt, R. K.; Holland, E.; Mohammed, U.; Paradine, S. M.; French, A. N.; Richter, C.; Bahar, A. M.; Witulski, B.; Wirth, T. Eur. J. Org. Chem. 2008, 5315.
[42] Farooq, U.; Schäfer, S.; Ali Shah, A.-U.-H.; Freudendahl, D. M.; Wirth, T. Synthesis 2010, 1023.
[43] Volp, K. A.; Harned, A. M. Chem. Commun. 2013, 49, 3001.
[44] Boppisetti, J. K.; Birman, V. B. Org. Lett. 2009, 6, 1221.
[45] Guilbault, A.-A.; Basdevant, B.; Wanie, V.; Legault, C. Y. J. Org. Chem. 2012, 77, 11283.
[46] Rodríguez, A.; Moran, W. J. Synthesis 2012, 44, 1178.
[47] Uyanik, M.; Yasui, T.; Ishihara, K. Angew. Chem. Int. Ed. 2013, 52, 9215.
[48] Uyanik, M.; Sasakura, N.; Mizuno, M.; Ishihara, K. ACS Catal. 2017, 7, 872.
[49] Uyanik, M.; Yasui, Y.; Ishihara, K. J. Org. Chem. 2017, 82, 11946.
[50] Jain, N.; Xu, S.; Ciufolini, M. A. Chem. Eur. J. 2017, 23, 4542.
[51] Molnár, I. G.; Gilmour, R. J. Am. Chem. Soc. 2016, 138, 5004.
[52] Scheidt, F.; Schäfer, M.; Sarie, J. C.; Doniliuc, C. G.; Molloy, J. J.; Gilmour, R. Angew. Chem. Int. Ed. 2018, 57, 16431.
[53] Ochiai, M.; Takaoka, Y.; Masaki, Y. J. Am. Chem. Soc. 1990, 112, 5677.
[54] Ochiai, M.; Kitagawa, Y.; Takayama, N.; Takaoka, Y.; Shiro, M. J. Am. Chem. Soc. 1999, 121, 9234.
[55] Deng, Q.-H.; Wang, J.-C.; Xu, Z.-J.; Zhou, C.-Y.; Che, C.-M. Synthesis 2011, 18, 2959.
[56] Quideau, S.; Lyvinec, G.; Marguerit, M.; Bathany, K.; Ozanne-Beaudenon, A.; Buffeteau, T.; Cavagnat, D.; Chénedé, A. Angew. Chem. Int. Ed. 2009, 48, 4605.
[57] Bosset, C.; Coffinier, R.; Peixoto, P. A.; Assal, M. E.; Miqueu, K. M.; Sotiropoulos, J.-M. Pouységu, L.; Quideau, S. Angew. Chem. Int. Ed. 2014, 53, 9860.
[58] Companys, S.; Peixoto, P. A.; Bosset, C.; Chassaing, S.; Miqueu, K.; Sotiropoulos, J.-M.; Pouységu, L.; Quideau, S. Chem. Eur. J. 2017, 23, 13309.
[59] (a) Brenet, S.; Berthiol, F.; Einhorn, J. Eur. J. Org. Chem. 2013, 8094.
(b) Brenet S.; Minozzi, C.; Clarens, B.; Amiri, L.; Berthiol, F. Synthesis 2015, 47, 3859
[60] Dohi, T.; Sasa, H.; Miyazaki, K.; Fujitake, M.; Takenaga, N.; Kita, Y. J. Org. Chem. 2017, 82, 11954.
[61] Levitre, G.; Dumoulin, A.; Retailleau, P.; Panossian, A.; Leroux, F. R.; Masson, G. J. Org. Chem. 2017, 82, 11877.
[62] Xue, J.-H.; Zhou, Q.-L. Acta Chim. Sinica 2014, 72, 778(in Chinese). (谢建华, 周其林, 化学学报, 2014, 72, 778.)
[63] Dohi, T.; Maruyama, A.; Takenaga, N.; Senami, K.; Minamitsuji, Y.; Fujioka, H.; Caemmerer, S. B.; Kita, Y. Angew. Chem. Int. Ed. 2008, 47, 3787.
[64] Dohi, T.; Takenaga, N.; Nakae, T.; Toyoda, Y.; Yamasaki, M.; Shiro, M.; Fujioka, H.; Maruyama, A.; Kita, Y. J. Am. Chem. Soc. 2013, 135, 4558.
[65] Yu, J.; Cui, J.; Hou, X.-S.; Liu, S.-S.; Gao, W.-C.; Jiang, S.; Tian, J.; Zhang, C. Tetrahedron:Asymmetry 2011, 22, 2039.
[66] Ding, Q.; He, H.; Cai, Q. Org. Lett. 2018, 20, 4554.
[67] Wang, Y.; Yuan, H.; Lu, H.; Zheng, W.-H. Org. Lett. 2018, 20, 2555.
[68] Murray, S. J.; Müller-Bunz, H.; Ibrahim, H. Chem. Commun. 2012, 48, 6268.
[69] Ogasawara, M.; Sasa, H.; Hu, H.; Amano, Y.; Nakajima, H.; Takenaga, N.; Nakajima, K.; Kita, Y.; Takahashi, T.; Dohi, T. Org. Lett. 2017, 19, 4102.
/
| 〈 |
|
〉 |